V-Safe Part 4: CDC Designs V-Safe to Assure Harms Are Hidden in Free-Text Fields So It Can Control What Becomes Public, Including Limiting the Harms Submitted to VAERS
Fourth part of an incredible story that shows just how broken our public “health” apparatus is: very, very broken.
If you have not already read Parts 1, 2 and 3 of the v-safe saga, please read those first!
Let’s start with the interplay of v-safe and VAERS and the question of whether v-safe is better (or at least has the potential to be better) than VAERS? It is – absolutely.
Unlike VAERS, the data in v-safe is gathered from a known and quantifiable universe of individuals. In fact, v-safe has precisely 10,108,273 registered users as of August 2022. These users are asked to answer the same questions. By aggregating answers to identical questions in v-safe, the rate of an adverse reaction can be calculated. That is not possible with VAERS.
For example, of the 10,108,273 registered v-safe users, 782,913 reported needing medical care after vaccination. So, you divide 782,913 by 10,108,273 and, poof, you now know that 7.7% of all registered v-safe users sought medical care at least once following vaccination. You can see these numbers for yourself on theICANv-safe dashboard:
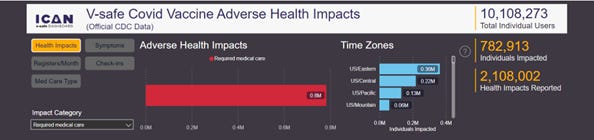
(Note that if the approximately 528,381 individuals that registered but never completed a single health check-in were excluded from the 10,108,273 v-safe users it would make the denominator smaller and therefore the 7.7% percentage greater – to approximately 8.2%.)
VAERS, on the other hand, is a passive surveillance system and that type of math is not possible because there is no known number of VAERS “users” to use as a denominator.
(For what it’s worth, VAERS has two advantages over v-safe. First, a user can submit an unlimited amount of information to VAERS about an adverse health impact. That is not true of v-safe which limits its free-text fields, where users can report symptoms, to 250 characters. Second, pursuant to federal law, a large portion of the deidentified information submitted to VAERS is promptly made public – although there are issues with this system. That is not true of v-safe.)
There are a lot of indications, including common sense, that v-safe users were less likely to report to VAERS, having already reported to v-safe. A key question is whether this was just an unintended consequence of v-safe.
Had v-safe not existed, there is a high likelihood that more people would have reported to VAERS and, again, theoretically, the public would have access to that data. We may never know the content or precise number of free-text field submissions in v-safe, which are still hidden from public view (although we continue to fight to obtain this data), whereas we would have access to public-facing VAERS reports.
The CDC knew that v-safe users would not also report to VAERS. This is reflected in the CDC’s public guidance regarding v-safe. First, the CDC treated VAERS as the alternative if v-safe was not available. For example, ittells members of the public that, “If you cannot participate in v-safe, you can submit reports of adverse events following vaccination to the Vaccine Adverse Event Reporting System (VAERS).” Or, if a user misses a v-safe check-in and still wants to report, then they are to use VAERS.
Second, the CDC claimed it would follow-up with individuals making certain types of reports to v-safe and help them submit a VAERS report “if appropriate.” CDC’s January 28, 2021 v-safeprotocol provided that any adverse health impact reported should result in a phone call. Presumably not realizing the volume of reports that would entail (millions!), by the next version of the v-safe protocol, issued May 20, 2021, the CDC limited active follow-up calls to “recipients reporting a significant, medically attended health impact during v-safe health check-ins.” This is a far narrower group that would receive contact from CDC and, again, the agency would only make a VAERS report “if appropriate.” In this way, the CDC has now made itself the middleman between vaccinated people and VAERS.
The biggest issue is that this plan to operate as a middleman to assist with VAERS reporting was seemingly theater. The “if appropriate” language likely means the CDC only made VAERS reports when dealing with injuries that were mandated to be reported to VAERS, and that is an extremely limited list of events. You can see the listhere.
Meaning, instead of reports being made directly to VAERS, the CDC created a limited list of conditions for which it would even call v-safe users and then only assisted with a VAERS report if it deemed it appropriate. This, no doubt, resulted in a large number of reports of injuries that would have otherwise been reported to VAERS never making it into that system.
CDC further “rigged” v-safe by relegating reported harms to free-text fields that kept them out of VAERS and hidden from the public.
In Part 2 of this Substack series, we discussed how the CDC failed to include check-the-box options for the serious harms it labeled “Adverse Events of Special Interest” and listed in a chart under the header “Prespecified Medical Conditions.” The adverse events of special interest listed in this chart included myocarditis, pericarditis, acute myocardial infarction, stroke, GBS, and transverse myelitis, among other events. This chart was in version 2 of the CDC’s v-safe protocol from January 28, 2021. You may recall that v-safe was launched in December 2020.
We have now obtained a copy of version 1 of the CDC’s v-safe protocol dated November 19, 2020, before v-safe was launched. And version 1 shows the precise chart from version 2 of the CDC’s v-safe protocol.Hereis a copy of version 1 of the protocol and here is the relevant chart:

Despite the foregoing, v-safe was launched without, and was never updated, to include any check-the-box fields for these conditions.
Had the CDC included the check-the-box options for these adverse events, it could have clearly calculated a rate for each harm for the 10 million v-safe users. For example, if 400,000 reported myocarditis, then that would be around a 4% reported rate for this condition. The CDC, however, chose not to include these harms as check-the-box options. It instead relegated them to only potentially be captured in free-text fields!
The CDC then engaged in the shell game of only calling certain v-safe users and then only making VAERS reports when it deemed doing so appropriate. In this way, it hid the adverse events of special interest in the free-text fields and kept them, no doubt, mostly buried and outside of public view by not having them all go into VAERS.
While the CDC avoided being able to easily calculate a rate of harm by not using check-the-box fields for adverse events of special interests, the hope would be that it at least timely followed up with v-safe users reporting serious harms in the free-text fields to learn more about their injuries. This way it can assess what harms the vaccine was causing and quickly address those harms. After all, the CDC claimed v-safe is a “real time” surveillance system for Covid-19 vaccines “so scientists can quickly study them and determine if there is a safety concern with a particular vaccine.”
Well, the story of one Ph.D. who got vaccinated early to set an example and to encourage her students to do the same reflects otherwise.
Despite clearlyreportinga significant medically attended health impact numerous times — which included brain swelling, toxic reaction, abnormal heart rhythm, numbness and tingling, high fever, chest pain, joint pain, light sensitivity, dizziness, impaired balance, nausea, vomiting, and lethargy — and begging for someone to help her in her v-safe reports, and trying to call v-safe herself, the CDC did not call her until over 200 days after she reported her serious injury!
Here, for example, is what shesubmittedto v-safe just a couple of weeks after her vaccination, which included the plea “Help me!”:
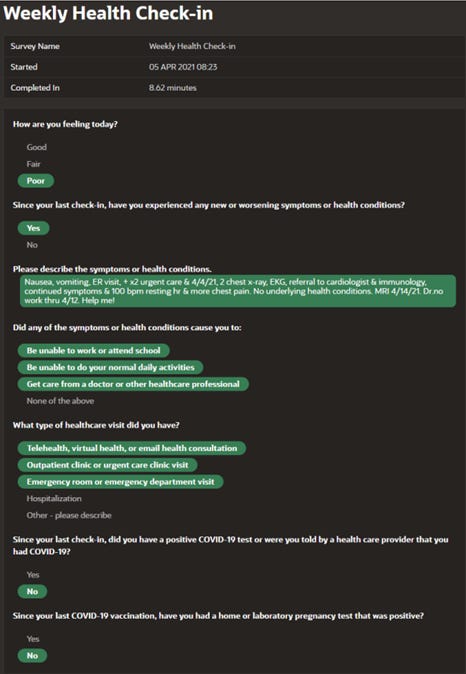
Her pleas for help continued with every health check-in over the next few months which detailed serious and worsening medical condition from the vaccine and still no follow-up from the CDC. See her 6-month check-in below where she notes “still no response from CDC”:
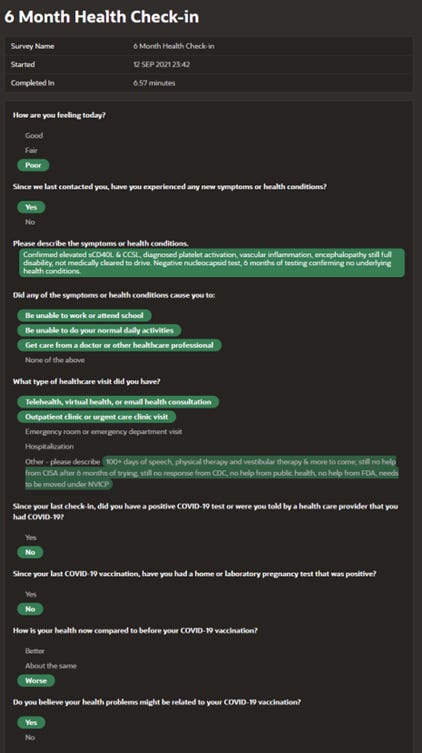
When the CDC did finally call her over 200 days after pleading for help, the CDC’s first question was to ask the date she recovered. She explained she did not recover, was still on full disability, still not cleared to drive, still not cleared to walk more than a limited number of steps per day, etc. It was clear the CDC representative had not even read her v-safe reports and had no interest in actually assessing what had happened to this Ph.D. that was initially strongly advocating everyone get the shot!
Makes one wonder how long people who reported “just” being hospitalized or seeking emergency room treatment, without any pleas for help or descriptions of symptoms and medical testing, needed to wait to get a call from the CDC. And it is also clear that when a call finally arrived, it was not about improving safety of these products.
So much for the CDC’s “real time” surveillance “so scientists can quickly study them and determine if there is a safety concern with a particular vaccine.”
That’s all for today. Next part of the v-safe saga will tell the story of the fight to get the check-the-box v-safe data and a deeper dive into what it shows.
Until then, please remember: the stop gap that prevents the freight train of issues with government mandated medicine smashing into you and your family is the ability to say “no” without penalty. The importance of that right – to never be mandated or coerced to receive a medical product – should already be clear, but if not, the next few v-safe parts should hopefully drive it home.
