
https://www.defense.gov/News/Contracts/Contract/Article/612903/
Vaccine Co. LLC, Frederick, Maryland (W81XWH-15-D-0037); PPD Development LP, Wilmington, North Carolina (W81XWH-15-D-0038); Leidos Inc., Reston, Virginia (W81XWH-15-D-0039); and Tasc Inc. [ENGILITY CORPORATION], Andover, Massachusetts (W81XWH-15-D-0042), were awarded a $501,000,000 order dependent contract with options for medical research.
Funding and work location will be determined with each order, with an estimated completion date of July 30, 2020.

April 10, 2013 – Alchem Laboratories Corporation is Teaming with Nanotherapeutics in a Department of Defense Contract for Advanced Development and Manufacturing of Medical Countermeasures.
Nanotherapeutics, Inc a biopharmaceutical company located in Alachua FL, announced that it has been awarded a Department of Defense (DOD) contract to establish Medical Countermeasures Advanced Development and Manufacturing (MCM ADM) capability dedicated to meet the needs of DOD.
https://inside.battelle.org/blog-details/bringing-down-barriers-to-medical-countermeasure-development Bringing Down Barriers to Medical Countermeasure Development (battelle.org)
In 2013, the DoD contracted with Nanotherapeutics Inc. to create the organization as a shared resource for companies working on the medical countermeasures the DoD needs. The ADMc has state-of-the-art manufacturing facilities for vaccines and biologics to help smaller companies scale up from R&D to manufacturing.
The ADMc also is prepared to support the production of
Battelle has partnered with Nanotherapeutics Inc. [http://web.archive.org/web/20130327000141/http://www.nanotherapeutics.com/] to help connect innovative biotech companies with the resources they need for successful development of medical countermeasures. The new collaboration brings together core research and manufacturing capabilities for development of new vaccines and therapies for chemical and biological weapons.
Robert W Malone PROFESSIONAL EXPERIENCE
http://web.archive.org/web/20130615133855/http://www.nanotherapeutics.com/ Welcome to Nanotherapeutics | Nanotherapeutics (archive.org)
James D. Talton, Ph.D., President and Chief Executive Officer – Dr. Talton is a co-founder, President, and CEO at Nanotherapeutics.
James D. Talton, Ph.D., is the President and CEO of Alchem Laboratories Corporation. He also is President of Nanopharmaceutics, Inc…
Robert W. Malone, MD, MS:


Alchem also announced recent key appointments to its management team. The appointments include Robert W. Malone M.D., M.S… Dr. Malone has over thirty years of research and development experience (bench to bedside) in the areas of pre-clinical discovery research, clinical trials, all phases of drug development, vaccines, gene therapy, bio-defense medical countermeasure development, pathology, molecular biology, virology and immunology.
https://www.latimes.com/nation/la-xpm-2013-nov-23-na-biodefense-spending-20131124-story.html Pentagon makes costly foray into biodefense drug business – Los Angeles Times (latimes.com)
WASHINGTON — Despite intense pressure to hold down federal spending, the Defense Department is launching a high-priced effort to create its own production pipeline for vaccines and biodefense drugs — an initiative that defies the advice of government-hired experts and duplicates what another agency is doing.
Construction began in late October on a plant in north Florida that will produce flu vaccine and specialized medicines for the Pentagon to protect military personnel against germ warfare agents.
On March 20 of this year [2013], the Defense Department awarded its initial manufacturing-facility contract, worth as much as $358.9 million, to Nanotherapeutics Inc. of Alachua, Fla., about 20 miles north of Gainesville.
Though the 165,000-square-foot plant is to be built and owned by Nanotherapeutics, it will be used exclusively for Pentagon-directed manufacturing.
The DoD MCM ADM and the business arrangements that support it were created by DoD’s Joint Project Manager Medical Countermeasure Systems (JPM-MCS). JPM-MCS’ mission is to provide U.S. military forces — and the nation — with safe, effective, and innovative medical solutions to counter chemical, biological, radiological, and nuclear threats.
The ceremony celebrates the groundbreaking of the 30-acre NANO-ADM Center, being constructed through privately secured financing to fulfill the contract awarded to Nanotherapeutics by the U.S. Department of Defense (DOD) earlier this year. Nanotherapeutics, as the prime contractor for the contract, is responsible for ensuring that all the core services necessary to establish a Medical Countermeasures Advanced Development and Manufacturing (MCM ADM) capability are provided to the DOD.
As the leader of the NANO-ADM Center, Nanotherapeutics will facilitate MCM product development and domestic vaccine manufacturing and surge capacity both in-house and through its teaming network. Upon completion of the NANO-ADM Center, the DOD and other third parties will have access to a state-of-the-art, ground-up development incorporating single-use equipment in a one-of-a-kind, 165,000 square-foot facility. The NANO-ADM Center will integrate new biomanufacturing technologies with existing capabilities, enabling the development of both small molecule and biologic products. The Center will include space for vector development, quality control, a development pilot plant, manufacturing core, warehouse, as well as office/administration and utilities. Over time, the NANO-ADM Center will also offer its services and capabilities in MCM and infectious disease manufacturing to broader customer bases, including other U.S. federal agencies, including the U.S. Department of Health and Human Services, as well as industry.
[https://cdn.who.int/media/docs/default-source/immunization/covid-19/16-june-22080-sinopharm-vaccine-explainer-update.pdf?sfvrsn=ad833095_1&download=true] – a cell culture-based platform for vaccine production which Nanotherapeutics acquired from Baxalta, formerly Baxter International’s BioScience division. Financial terms of the agreement were not disclosed.
In 2010, Takeda and Baxter entered into an agreement in which Takeda licensed from Baxter certain exclusive rights to the technology for the development of pandemic and seasonal influenza vaccines for the Japanese market. Takeda’s cell culture-based H5N1 and prototype vaccine for pandemic influenza was developed on this platform and was approved in Japan in March 2014. Takeda is currently developing a cell-based seasonal flu vaccine (TAK-850) on the same platform for use in Japan.
“Today’s agreement reinforces Takeda’s commitment to its global vaccine business and our goal of reaching as many people as possible with vaccines that address important unmet needs in global public health.”
https://www.biospace.com/article/releases/battelle-and-nanotherapeutics-form-alliance-to-speed-up-process-of-industry-bringing-medical-countermeasures-to-market-/ Published: Apr 05, 2017
Focus is on vaccines and therapeutics to counter Chemical, Biological, Radiological and Nuclear (CBRN) threats to U.S. Armed Forces
COLUMBUS, Ohio–(BUSINESS WIRE)–Battelle and Nanotherapeutics, Inc. [http://web.archive.org/web/20140517192815/http://www.nanotherapeutics.com/] announced today that the organizations have entered an alliance to bring together core research, development, test and evaluation (RDT&E) and manufacturing capabilities needed to expedite the development of medical countermeasures urgently required by the Department of Defense (DoD) to protect deployed military forces from CBRN threats.
Nanotherapeutics is a contract development and manufacturing organization (CDMO) that operates a U.S.-based 183,000 square foot, state of the art, single-use, multi-purpose, multi-product, BSL-3 capable facility offering clients extensive capabilities, including a pilot facility for performing optimization of upstream, downstream and formulation functions, bulk cGMP manufacturing, and analytical development for proteins, antibodies, viral vaccines and gene therapy drug products. The Company also owns a second, Czech Republic-based, 165,000 square foot, 6,000-liter capacity BSL-3 facility for manufacturing vaccines using its proprietary Vero cell platform.
PharmAthene and Nanotherapeutics, a privately-held biopharmaceutical company, have formed a Strategic Alliance to advance the development of certain medical countermeasures for the U.S. biodefense market.
“There is a real concern that the nation is not as prepared for manmade and naturally occurring biothreats as it can be and should be. We believe our alliance with Nanotherapeutics will accelerate the development and availability of promising medical countermeasures,” commented Eric I. Richman, President and Chief Executive Officer of PharmAthene.
“By combining our complementary resources and expertise on select programs, we hope to accelerate the development of urgently needed medical countermeasures.”
In 2013, the DoD selected Nanotherapeutics as its exclusive prime contractor through a competitive process. The 10-year, $359 million contract provides for the establishment of a Medical Countermeasures Advanced Development and Manufacturing Center (NANO-ADM) to provide more rapid development and manufacture of bulk vaccines and biologics for the DoD and potentially other government and commercial partners.
“We look forward to utilizing Nanotherapeutics’ manufacturing capabilities for this program…” said Dr. James Talton, President and Chief Executive Officer of Nanotherapeutics.
https://www.manufacturing.net/operations/news/13095313/strategic-alliance-formed-to-advance-national-biodefense-programs By The Associated Press, Sep 8, 2014
PharmAthene, Inc. and Nanotherapeutics, Inc., a privately-held biopharmaceutical company, have announced that they have formed a Strategic Alliance to advance the development of certain medical countermeasures for the U.S. biodefense market.
In 2013, the DoD selected Nanotherapeutics as its exclusive prime contractor through a competitive process. The 10-year, $359 million contract provides for the establishment of a Medical Countermeasures Advanced Development and Manufacturing Center (NANO-ADM) to provide more rapid development and manufacture of bulk vaccines and biologics for the DoD and potentially other government and commercial partners. Construction of the NANO-ADM Center commenced in October 2013 and is expected to be completed in 2015.
https://en.wikipedia.org/wiki/Ology_Bioservices
Ology Bioservices (previously named Nanotherapeutics, Inc.) is a private, American biopharmaceutical company headquartered in Alachua, Florida. The company was founded with research in nanometer-scale particle technology to develop new drug delivery technologies and increase the efficacy of existing drugs. In 2016, the company changed their strategic focus and became a biologics contract development and manufacturing company (CDMO) specializing in the manufacturing of vaccines, monoclonal antibodies, recombinant proteins, virus and nucleic acids.
Nanotherapeutics was a product of the Sid Martin Biotechnology Incubator of Alachua, Florida. It was established in 2000 as Nanocoat Technologies by James Talton. The company changed its name to Nanotherapeutics in 2002.
At the end of 2014, Baxter sold its vaccine production technology based on Vero cell culture to Nanotherapeutics. The sale included developed vaccines for H5N1, H1N1 and seasonal influenza, as well as a number of investigational vaccine programs.
Historically, Nanotherapeutic’s product development efforts were based on utilization of nanometer-scale particle technology. This placed at least some of its products in the category of medical devices, such as NanoFuse DBM, approval of which can take advantage of the FDA 510(k) expedited review route.
A senior Defense Department official, Arthur T. Hopkins, told a House Armed Services subcommittee in February 2016 that the military “needs the facility to rapidly develop and produce vaccines.’’
That year, at an initial cost of $204 million in public funds, the 180,000 square-foot facility opened in Alachua in partnership with a private company now called Ology Bioservices Inc. Since then, the Defense Department has spent about $66 million to maintain the readiness of equipment there, federal records show.
https://globalbiodefense.com/2019/04/15/ology-bioservices-awarded-dod-contract-for-countermeasures-against-bioweapons/ by Global Biodefense Staff, April 15, 2019
The Department of Defense (DOD), through its Platforms for Rapid Integrated Solutions for Medical Countermeasures (PRISM) office, has awarded Ology Bioservices two contracts valued at $135.4 million.
The first contract award is for the “Development and Utilization of a Monoclonal Antibody Platform Prototype for Development of Monoclonal Antibodies as Medical Countermeasures Against Threats of Interest.
PRISM also awarded Ology Bioservices a $5.1 million, 30-month contract entitled, “Developing, Establishing and Exercising Plasmid DNA Manufacturing Capabilities at the DOD Advanced Development and Manufacturing Facility.”
Under this program, Ology Bioservices will develop and deliver a prototype manufacturing platform for the CGMP production of nucleic acids, primarily plasmid DNA for use as both Drug Substance and high-quality intermediate. Plasmid DNA is important, primarily for its use in DNA vaccines and gene therapy. The aim of the project is to more rapidly and efficiently deliver biodefense medical countermeasures to warfighters, with reduced developmental risk.
This contract also includes optional components that would facilitate the development of capabilities for manufacturing RNA, another area of potential value to the U.S. government and commercial clients for various uses, including RNA vaccines.
Ology Bio, based in Alachua, Florida, develops and manufactures drugs and biologics for commercial customers as well as the U.S. government, with more than $1.8 billion in government contracts awarded.
August 19, 2014 09:13 AM Eastern Daylight Time
ALACHUA, Fla.–(BUSINESS WIRE)–Nanotherapeutics, Inc., an integrated biopharmaceutical company with a focus on development and manufacturing, announced today the opening of a new, 4,800 square foot office, located at 8490 Progress Drive, Suite 450, Frederick, MD.
Nanotherapeutics’ new office is just five miles from Fort Detrick, which houses five cabinet level agencies, including the Department of Defense, as well as centers for cancer and AIDS research, including the National Cancer Institute. The office is also approximately 50 miles from Washington DC, providing easy access for Nanotherapeutics’ U.S. Government clients.
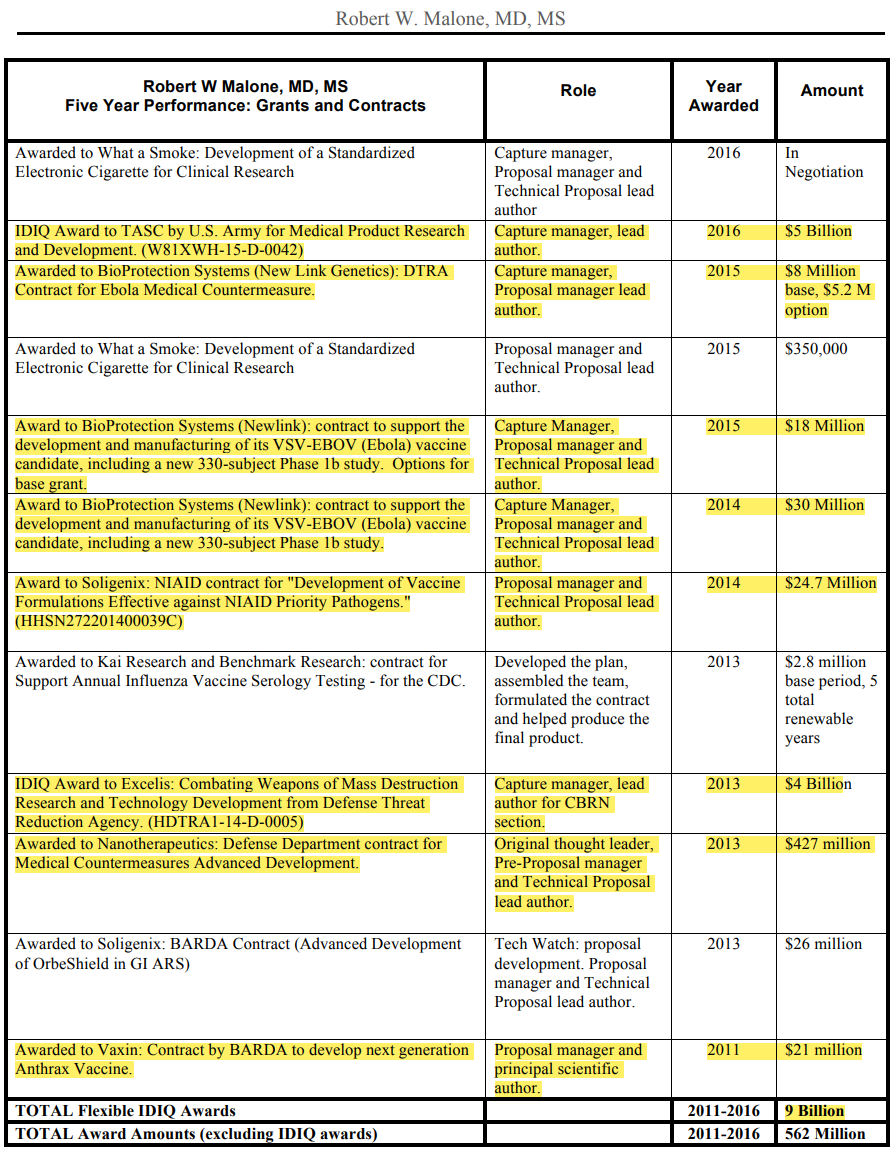
Source: ROBERT W MALONE – by OUTRAGED HUMAN – OUTRAGED’s Newsletter
