A lot of attention has been dedicated to the DOD-Pfizer contracts for mass poisoning injections falsely marketed as “vaccines”, and I have received many requests to cover Moderna contracts in more detail. Similarly to Pfizer, Moderna contracted with the DOD for a “manufacturing demonstration”. In parallel, they contracted with HHS/BARDA for “vaccine” R&D. This is different from Pfizer’s contracts where R&D and clinical trial activities where explicitly out of scope. Also differently from Pfizer and other covid contracts, Moderna’s contracts did not utilize Other Transaction Authority (OTA) contracting method. This does not seem to change the product liability, as Moderna’s poison is co-owned/co-developed with the US Government and is covered by the PREP Act like all other “covid countermeasures”.
As a brief background, Moderna was founded in 2011, financially and scientifically failed by 2016, and then was “rescued” from the trash heap by the conspicuously generous funding from the US Government. The government-military funding had been pouring into Moderna even before 2016. In October 2013, DARPA awarded Moderna nearly $25 million to research and develop potential mRNA compounds under DARPA’s Autonomous Diagnostics to Enable Prevention and Therapeutics (ADEPT) program: Developing technologies to rapidly detect and respond to threats from natural and man-made diseases and toxins. Back in March 2013, Moderna received funding from DARPA – and the second round of funds was to be used, according to press release, primarily to support vaccine and antibody development to protect against chikungunya infections. Moderna also received $955 million from BARDA – Biomedical Advanced Research and Development Authority to develop a Covid-19 vaccine based on mRNA technology in collaboration with the NIH.
The FDA’s January 30, 2022 “Summary Basis for Regulatory Action SPIKEVAX” document reveals the following timeline for Moderna’s Covid-19 injection product and the two IND numbers:
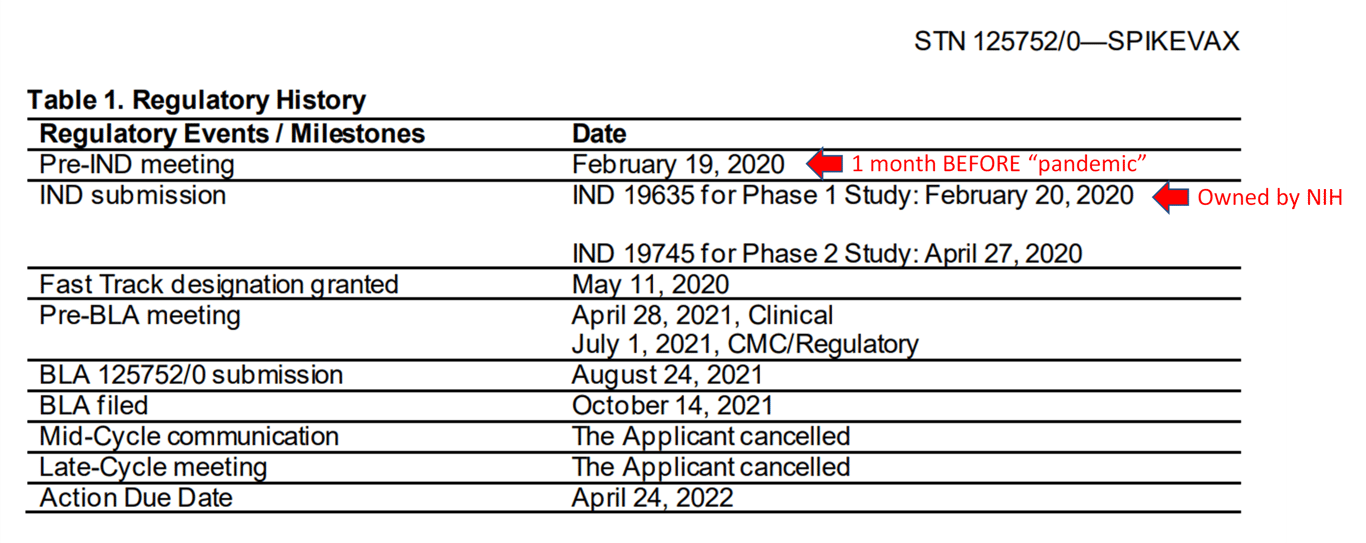
According to this timeline, the product has two sponsors/owners (holders of the Investigational New Drug application), one of whom is a division of the NIH at the time organizationally reporting to Anthony Fauci. The date of the pre-IND meeting for SPIKEVAX is February 19, 2020, the IND submission for NIH IND is the following day, February 20, while Moderna’s own IND was submitted on April 27, 2020.
According to the CDC, as of January 11, 2020, Chinese health authorities said they’ve identified more than 40 human infections as part of this outbreak that was first reported on December 31. The World Health Organization announced the preliminary identification of the novel coronavirus on January 9. The record of Wuhan-Hu-1 includes sequence data, annotation, and metadata of a virus genomic sequence from a case that occurred approximately two weeks prior.
This raises several questions. Some of these questions have been already answered, at least in part:
- How was it possible for the NIH/Moderna to have the pre-IND meeting for a Phase 1 human clinical trial scheduled with the FDA for a vaccine product a month before the “covid pandemic” was declared? Preparation for a pre-IND meeting is typically a several months long labor-consuming process.
- What is the precise commercial and legal arrangement between Moderna and NIH regarding SPIKEVAX? Ownership of the IND is both legal and commercial matter, which in the case of public-private partnership must be transparently disclosed.
- Does NIH financially benefit from sales of Moderna product? Who at NIH specifically? Note: this question has been answered at least in part, as recently Moderna paid out $400M in royalties to the US Government and Jason McLellan at Dartmouth, and to Dartmouth itself.
- Does forcing vaccination with the Moderna product via mandates, government-funded media campaigns, and perverse government financial incentives to schools, healthcare system, and employers represent a significant conflict of interest for the NIH as a financial beneficiary of these actions? Note: per above, YES.
- “Vaccine” contract and amendments that specifies R&D projects that the US Government ordered and paid for. Note that in Pfizer’s case no R&D activities were ordered or paid for by the US Government, as these were excluded from the scope of contract.
- “Manufacturing” contract(s) that, similar to all other vax contracts ordered a large scale manufacturing demonstration. This is similar in scope to the Pfizer contracts.
Note on redactions. In both Moderna and Pfizer’s contracts many areas are redacted indicating a reason for redaction – the “redaction codes”. Redacted content has been given codes b (4) and b (6), standing for:
(b) (4) Disclosure of information that would affect the application of advanced technology in a U.S. weapons system,
and
(b) (6) Disclosure of information, including information of foreign governments, that would cause serious harm to relations between the United States and a foreign government or to ongoing diplomatic activities of the United States.
U.S. bioweapons technology in vaccines? With the potential to cause severe damage to U.S. diplomatic relations?
(“Manufacturing” contract will be reviewed in Part 2).
HHS/ASPR/BARDA-Moderna Contract 75A50120C00034. April 16, 2020:
This contract was signed on April 16, 2020. The signatories on behalf of Moderna are Stephane Bancel, CEO, and on behalf of the US Government is Wendell Conyers. The contract is 112 pages long. The contract was originally for ~$500M but later amended to ~$1B (July 2020).

The scope can be summarized as a “contract R&D services for the US Government”. The Government is ordering and paying for pre-clinical, Phase 2 and 3 clinical studies and related CMC (Chemistry, Manufacturing and Controls) development. This is different from Pfizer contract which specifically excluded preclinical, clinical trials and CMC part from the scope of the DOD contract. Some of the studies in Moderna contract – most of the animal experiments as well as the human Phase 1 trial, were to be performed by the Vaccine Research Center at the NIH. Why? I suppose, because they are nice and routinely do work for private corporations. That’s a joke, of course, the NIH co-own the product, get payouts from Moderna ($400M recently, split with Dartmouth), and then the government buys the product at inflated prices and forces it on all of us.
Back to the contract: Moderna gets paid for “pre-award activities” and to do certain R&D studies. The payment is not dependent on whether these projects succeed or fail – Moderna gets paid $500M-$1B (per July 2020 amendment), almost regardless of what happens. The amount of money is absolutely staggering for a company that had no success record, no products on the market or even in late phases of clinical development, and no scaled manufacturing at the time. It is not possible to create cGMP compliant, reliable and safe bio-pharmaceutical manufacturing at scale in a few months regardless of the amount of money thrown at it. Everyone involved in decision making in the US Government/DOD/BARDA knew this well but proceeded to playact the marvelous “cinderella” narrative for Moderna. There is also no information in the contract (or a reference to an attachment/appendix) on how exactly the sum of project budget was determined. The line items discussed below are extremely high level, almost comical for the amount of money in question, and even if these were unredacted, it would not provide anything informative.
Note: CLIN stands for Contract Line Item Numbers.
What is visible here is that ATI, aka “The Manager” of the DOD contracts made a tidy profit! This profit was guaranteed no matter what. Since it is likely calculated as a % of total contract, the more inefficient the work (more time/cost over-runs) the better it is for ATI.
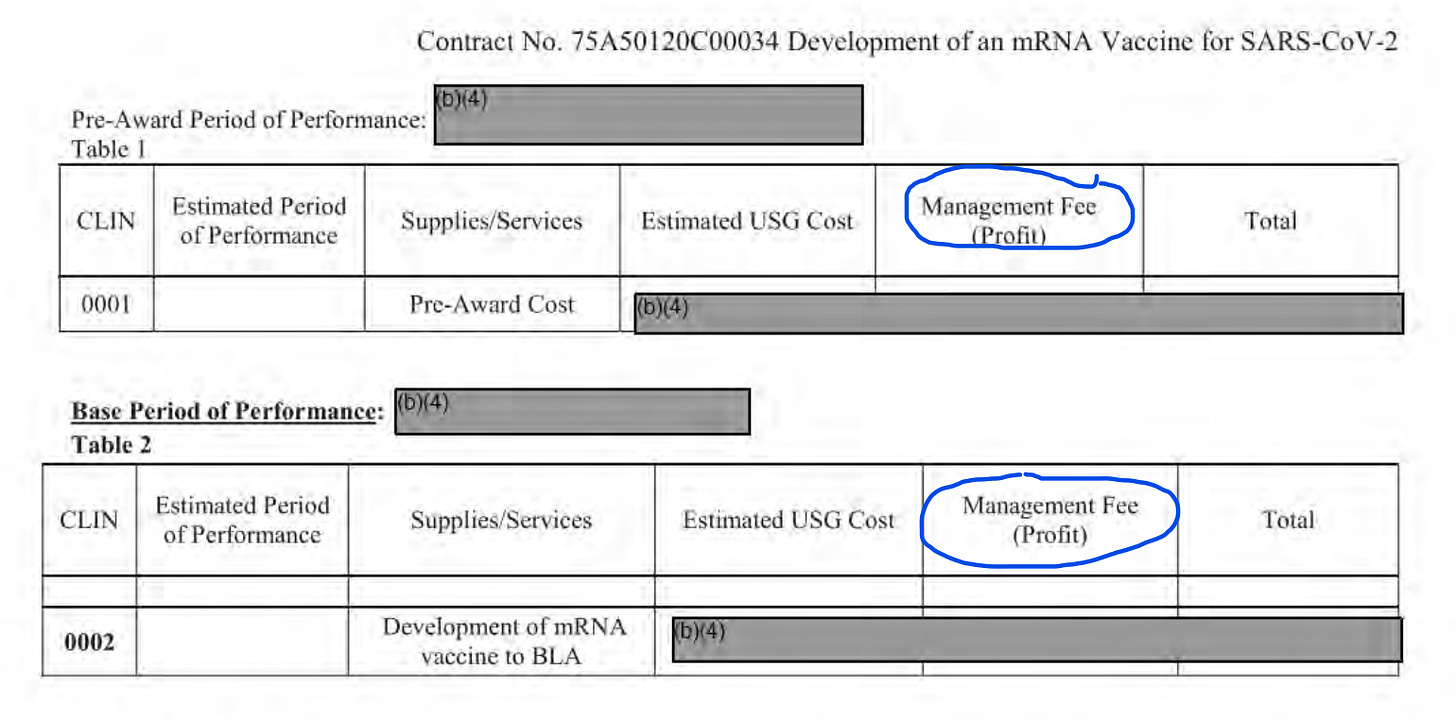
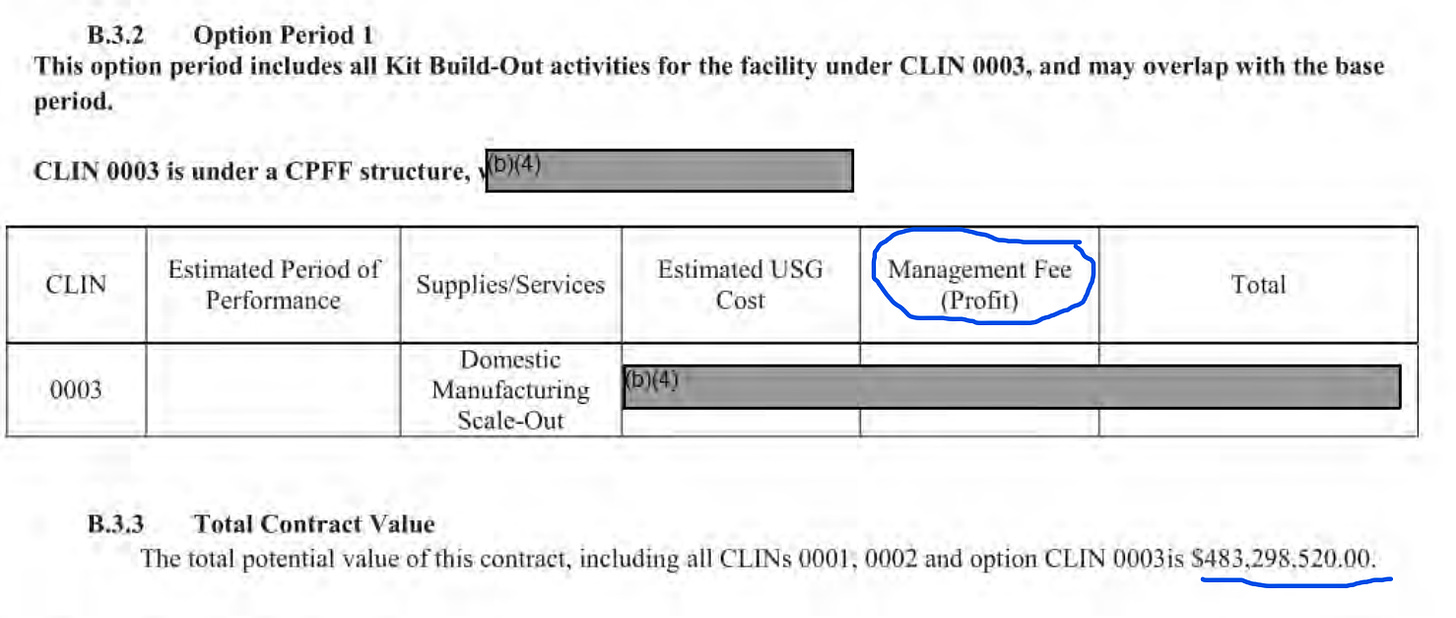
$500M-$1B split into 3 line items? Please! Can you see extremely suspect money transfers from the US Government to a “preferred” and co-owned corporate entity for vaguely defined activities under a panic scenario? Not to mention that by 2020 Moderna had already received $5 billion+ in investments and R&D grants (including another ~$500M from DARPA and BARDA) and still had no products! What was all this money for?
I am familiar with pharmaceutical R&D costs, including costs of late phase clinical trials, and in my very generous estimate, the program of studies ordered from Moderna by the US Government in this contract could not cost more than $50M. There wasn’t enough time to even try to spend this much – clinical trials are mostly based on the time of activities, they are service contracts. Building a factory (manufacturing scale out) could be expensive but that, too could not be done in the time that was allocated for it by this project. This contract does not include the cost of manufacturing of the shots, as those costs were in the second contract by which the government bought millions of shots.
Why was the US Government giving Moderna roughly 10X of the market rate for a limited and shoddy R&D program, while the US Government (VRC) was performing part of this program anyway?
The scope of the contract says “studies sufficient to demonstrate safety and efficacy” – however in real pharmaceutical R&D this is an unknown. We do not know how many attempts it will take to demonstrate safety and efficacy, especially for a really novel technology. We also do not know how long it will take. Real R&D is risky. Studies fail all the time. You cannot “order success”, especially not on a short timeline, and not from someone who had failed consistently during the previous 10 years! Therefore, this paragraph indicates that the product approval was independent of the success or failure of the studies being ordered. The studies listed (all details redacted with (b)(4)- the “weapons” redaction) were, in my professional opinion, woefully inadequate to attempt to demonstrate safety. Not even close.
There is a paragraph on p. 6 about formal “go/no-go” decision points at which presumably the Government can terminate this contract, but the dates of those reviews are redacted and no criteria for these decisions are specified.
Of course, the Government protected itself and Moderna from any liability under PREP Act!

The performance standard for this contract is “reasonable effort” – another indicator that the R&D program ordered through this contract is a sham:

“Efforts clauses” in contracts are most likely to be used where a party is unable to control an outcome, the parties are unable to predict if an outcome can be achieved, or a party simply is unwilling to guarantee an outcome. The obligation to use reasonable efforts, commercially reasonable efforts, or even best efforts does not generally mean that the promising party must be successful or take exhaustive measures to fulfill the obligation. Parties rarely bother to define what they mean by “best”, “commercially reasonable” or “reasonable” efforts, meaning that in a dispute they are leaving it to a court to determine not only whether the effort has been made but what the effort required in the first place. Courts have wide latitude in determining what the parties may have intended to be required.
So, in this case Moderna promises to do the studies that the Government pays for with a sort-of effort, but can’t make any firm commitments to success.
Here is the listing of the R&D projects that were ordered by the DOD from Moderna:
The detailed descriptions of studies in this section of the contract are heavily redacted with (b) (4) – i.e. “weapons” redaction.
I previously reviewed the completed Moderna non-clinical studies and many-faceted fraud that those studies involved here, yet again confirming that none of these pretend-R&D activities mattered for deploying the injections on the American public and global populations.
First study ordered – Development and Reproductive Toxicology (DART), the only GLP or Good Laboratory Practice-compliant study required in the entire program. Clearly, pregnant women and unborn babies were the target right from the start. The US Government wanted to see whether sterility and fetal death/malformations could be achieved after 5 doses or so, using the rat as a model. The Government ordered GLP-grade work to make extra sure that the results would be validated, i.e. reliable:

Next comes the reference to the studies to be done by the Vaccine Research Center of the NIH (VRC): “continues to support nonclinical…” = they are running animal experiments for Moderna in their labs. They also clearly know this mRNA concoction will induce “disease enhancement”, aka VAIDS, aka “breakthrough” covid, maybe more severe deadly covid in the injected population. They want this studied and confirmed!


Next R&D project is to “establish a surrogate of protection”.

I can talk for hours about surrogate endpoints in Pharma R&D, it is a huge topic. “Endpoints” in general are the measurements of success of failure of a research study and these must be declared upfront in the design of the experiment. In case of clinical trials, ideally the best endpoint is the clinical outcome in comparison to placebo in a randomized blinded experiment. “Surrogate” refers to a measurement that is not the outcome but is a well established predictor of an outcome. The rub is that the prediction of the outcome must be definitively established, and, in addition, the surrogate is always “less-than” the actual outcome measure. This is because it always has false-positives and false-negatives. Typically, it takes decades to establish a proper surrogate measure in clinical trials to replace an important outcome measure. I was involved in the establishing of the QT prolongation interval (on ECG/EKG) as a surrogate for life-threatening drug induced arrhythmias, and this one metric took between 1997 and 2006 to be definitively accepted, and subsequently, until 2010 to be accepted from computerized measurements.
Unlike proper pharmaceutical products, the vaccines are NEVER studied against actual health outcomes, i.e. prevention of a disease in question vs. true placebo control. For Covid-19 countermeasures that were not even vaccines, the situation was a total relaxation on the beach. Since none of the R&D studies actually mattered for approval, the Government even specified what surrogate endpoint is going to be “established” – neutralizing assays. Which are meaningless in demonstrating immunity in humans for many scientific reasons I won’t get into here, but especially because they are doing this in mice and monkeys. To emphasize once again, this is just a bunch of bs experiments to check boxes and collect truckloads of cash.
Next, they list the Phase 2 and 3 studies in humans, all descriptions of which are redacted with the same “weapons” redaction:

They are anticipating a scenario when there is no Sars-Cov-2 virus and no efficacy study is possible. Again all descriptions are redacted, since we are talking about state-of-the-art weapons systems.
Further, the Government is also ordering a lot (batch) consistency study, but we cannot know what it entails, as it is fully redacted. We know that lot-to-lot consistency for these injections was never achieved, again confirming none of these pretend studies had impact on the approval.
Finally a pediatrics study is on the list, also of course redacted with a “weapons” redaction. Let’s read this again – a study in children which is a secret because weapons.
I cannot prove this, but my guess is all of the redacted study descriptions in the contract were blank at the time of signing. Legally, Modernal didn’t have to do any of them for a military countermeasure, and it didn’t matter for the FDA fake-approval. The studies have been published since then, or otherwise released under FOIA, so I see absolutely no reason why these contract sections remain redacted. The only logical conclusion – they are hiding the fact these were simply blank sections signed by the US Government and Stephane Bancel. I would like to be proven wrong here.
The scope of the R&D program is about 10%-20% of the work a typical full development program for a very novel pharmaceutical technology would contain, i.e. highly inadequate to attempt to demonstrate efficacy, and especially safety, if undertaken in good faith.
Further, this section talks about “Regulatory” – simply listing activities such as IND preparation, filing, BLA filing, etc. Process and manufacturing validation are mentioned. Assays and stability study. All details of all these sections are redacted with (b)(4) – i.e. weapons system redaction.
On p.15 there is a section titled “Target Product Profile” – and it is completely redacted with the weapons system redaction as well. I wonder why? This is presumably written for a vaccine which is supposed to be safe and efficacious. In fact, there is even a WHO guidance for the covid vaccine product profile publicly available. So why is this one a secret?
Deliverables:
There is a lengthy summary of deliverables running from p.18 to p.29 – all of them are a variety of progress updates and reports. They simply specify a communications flow and data flow from Moderna to USG. No success criteria. No mention of compliance with anything. Same goes for the FDA submissions, reviews, approvals, or audits. It is simply a flow of information from Moderna to the Government that is required. In another place of the contract it is mentioned that the primary deliverable is a “licensed vaccine”, but as described above, that’s a given – it was going to be licensed no matter what before this contract was signed.
Many pages are dedicated to descriptions of reporting and data transfers.
While this contract is not under OTA, in my opinion, it is even less enforceable than the Pfizer contract with respect to the FDA regulatory compliance. There is very little language about compliance in it anyway. Some references are made to it, but nothing with any “teeth”. Unlike Pfizer’s contract for example, it doesn’t even say that the payment is subject to Moderna’s compliance with any of those regulations.
There is a clause about false and misleading information:

There is also an anti-terrorism clause, how nice!

And a bunch of other meaningless administrivia…
What is this contract for, really?
Imagine: you hire a contractor to “dig a ditch from this spot to 6 months from now” (it’s a Russian joke). You sign a contract that incudes paying the contractor for his previous activities which maybe-maybe-not had anything to do with your ditch. You had paid that same contractor $500M in the previous 5 years, for the same ditch, but he consistently failed. You will now pay another $500M guaranteed (almost), then in a few month expand it to $1B, because reasons. The ditch does not have to arrive anywhere in particular, or be any specific length, depth or width. You will dig a third of it yourself. The performance standard is “reasonable effort”. The deliverables are daily phone calls, meetings, progress reports, data tables and other papers describing the progress of the ditch. There is a manager of this project that takes a cut which is guaranteed as their profit. This makes a lot of sense to you somehow! Then the acid trip ends.
Let’s not overthink it. This funny contract is an account of plain looting by the Government and Moderna, together as co-conspirators against us, the powerless taxpayer. While government looting is not really new, this time we the taxpayers are also the target of the Globo-Fascist state-of-the-art weapons system consisting of randomly produced genetic-chemical poison, fear mongering, coercion and military-industrial strength bullshit. We are supposed to simply nod along with this production, and so many “experts” did just that!
**Source: Moderna contracts – Part 1 – by Sasha Latypova
