A reader asked me why I am “absolving Pfizer and Moderna from liability”? I am not, and I never did, and never implied such a thing. All pharmas, and not just pharmas – all “non-traditional” defense contractors (this includes 100’s of companies and academic institutions) taking blood money to develop, make, distribute and deploy biowarfare agents on civilians, including pregnant women, babies and children, and the service members under the pretense of “covid pandemic response” should be prosecuted for conspiring with the government to implement this domestic and international bioterrorism program.
American Domestic Bioterrorism Program
Research and organizing tool first posted April 28, 2022, subject to ongoing revision as new information comes to light. Last updated Jan. 09, 2023. Other formats: Sept. 2022 small-print PDF (67 pages); Sept. 2022 large-print PDF (101 pages); Nov. 2022…
For new readers who are not familiar with why I am using the “bioterrorism” term, I invite you to study the legal history and fact pattern:
- Under Stafford Act authority, President Trump declared a Public Health Emergency (PHE) on March 13, 2020 (Stafford Act, P.L. 93-288 as amended).
- Under a PHE, medical “countermeasures” are not regulated or safeguarded as normal pharmaceutical products (21 USC 360bbb-3(k): use of EUA-covered medical countermeasure (MCM) products, once designated as such by the Secretary of Health and Human Services (March 10, 2020, retroactive to February 4, 2020) “shall not be considered to constitute a clinical investigation.”
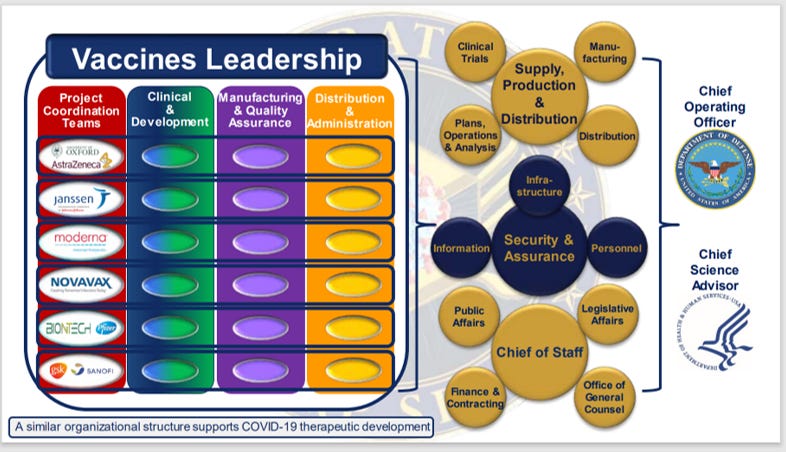
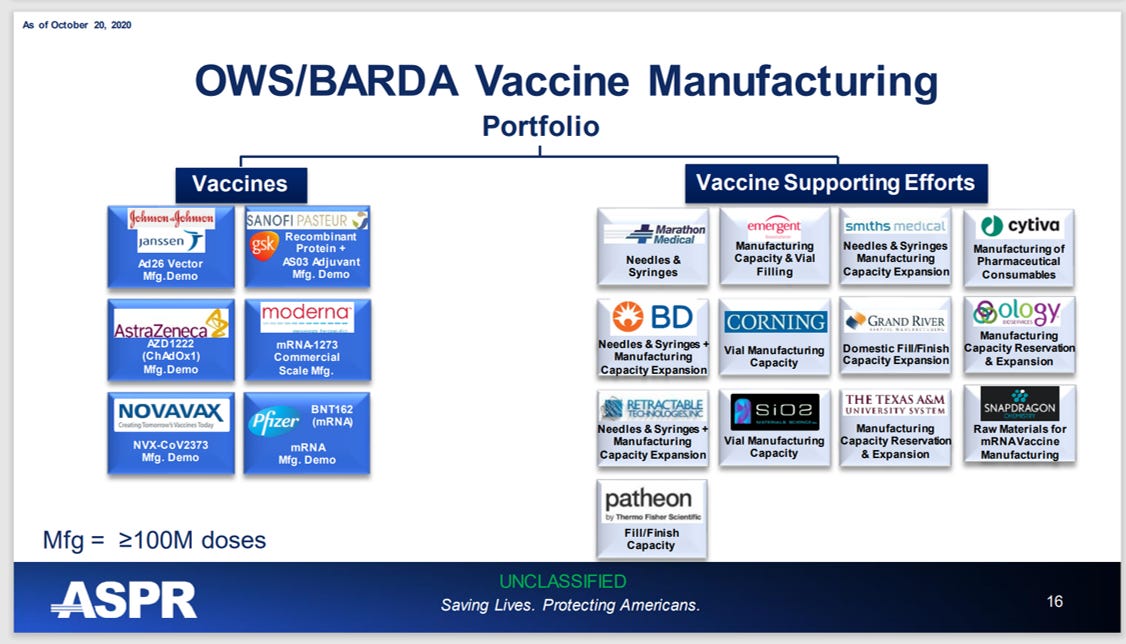
- According to Operation Warp Speed / Administration for Strategic Preparedness and Response (ASPR) reports, the United States Department of Defense (DoD) directed, oversaw and managed the development, manufacture and distribution of nearly all Covid countermeasures, largely utilizing DoD’s previously established network of military contractors andconsortia. Slides from Operation Warp Speed/BARDA, October 22, 2020:
- DoD, the Biomedical Advanced Research and Development Authority (BARDA) and the Department of Health and Human Services (HHS) contracted for Covid countermeasures, including “vaccines,” as “prototype demonstrations” of “large-scale manufacturing.”
- These agencies avoided nearly all relevant legal and transparency requirements by using Other Transaction Authority (OTA)contracts.
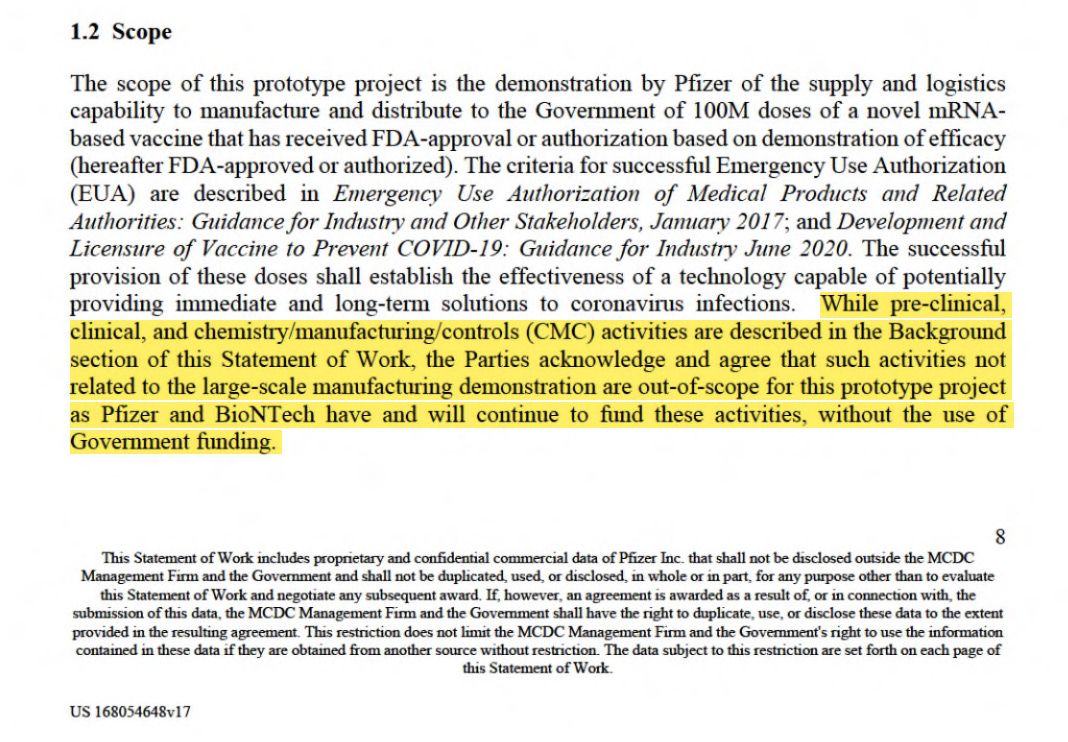
- Although the DoD/BARDA contracts refer to “safety and efficacy requirements” and mention cGMP compliance, these items are explicitly carved out asnot being paid for, and therefore not ordered(in any enforceable manner) by the U.S. Government.Statement excluding pre-clinical, clinical trials, and chemistry/manufacturing/controlsout of the scope of the DOD contract (from Pfizer’s ATI Technical Direction Letter). This statement is typical for all “vaccine” contracts:
- The contracts for countermeasures include a liability shield for manufacturers and contractors along the supply and distribution chains, under the PREP Act. See example PREP Act clause from Moderna contract. Note the statement at the end about this product being “both civil and military application” (p. 26here):
As prototypes under Emergency Use Authorization (EUA) during a PHE, Covid countermeasures neednotcomply with laws governing clinical trials, manufacturing quality, safety or labeling (21 USC 360bbb-3(k)).
The result: we have a chaotic mess of everything from sham injections that may be mostly just saline all the way to extremely dangerous/deadly shots, all of which are being distributed under the same product brands and labels.