Since the onset of the COVID-19 pandemic two years ago, hundreds of studies have examined the efficacy of dozens of drugs and other compounds in treating the disease. While the research is still ongoing, a sizable number of treatments have shown promise. A growing number of them have been authorized by the U.S. government, though virtually all of those approved sport hefty price tags.
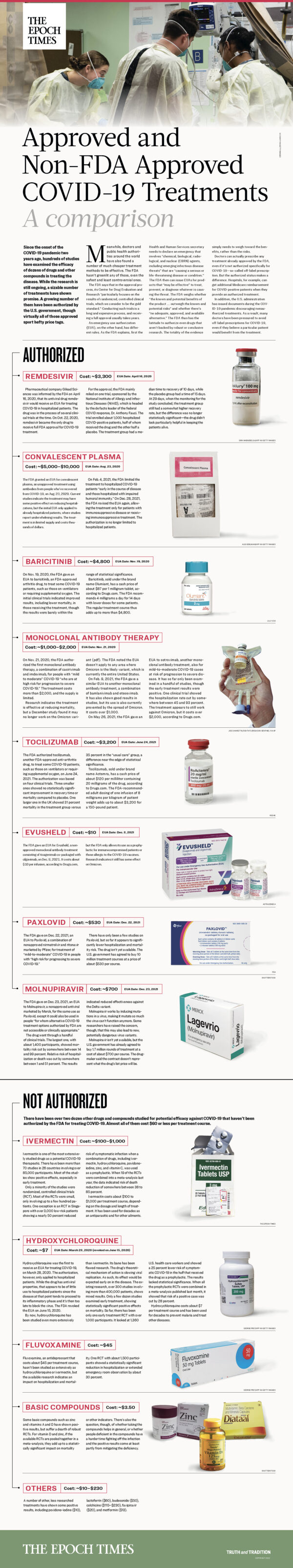
Meanwhile, doctors and public health authorities around the world have also found a number of much cheaper treatment methods to be effective. The FDA hasn’t greenlit any of those, even the safest and least controversial ones.
The FDA says that in the approval process, its Center for Drug Evaluation and Research “particularly focuses on the results of randomized, controlled clinical trials, which we consider to be the gold standard.” Conducting such trials is a long and expensive process, and receiving a full approval usually takes years.
An emergency use authorization (EUA), on the other hand, has different rules. As the FDA explains, first the Health and Human Services secretary needs to declare an emergency that involves “chemical, biological, radiological, and nuclear (CBRN) agents, including emerging infectious disease threats” that are “causing a serious or life-threatening disease or condition.” The FDA then can issue EUAs for products that “may be effective” to treat, prevent, or diagnose whatever is causing the threat. The FDA weighs whether “the known and potential benefits of the product … outweigh the known and potential risks” and whether there’s “no adequate, approved, and available alternative.” The FDA thus has the latitude to authorize even drugs that aren’t backed by robust or conclusive research. The totality of the evidence simply needs to weigh toward the benefits, rather than the risks.
Doctors can actually prescribe any treatment already approved by the FDA, even if it’s not authorized specifically for COVID-19—so-called off-label prescription. But the authorized status makes a difference. Hospitals, for example, can get additional Medicare reimbursement for COVID-positive patients when they provide an authorized treatment.
In addition, the U.S. administration has issued documents during the COVID-19 pandemic discouraging nonauthorized treatments. As a result, many doctors have been pressured to avoid off-label prescriptions for COVID-19, even if they believe a particular patient would benefit from the treatment.
Source: INFOGRAPHIC: Approved and Non-FDA Approved COVID-19 Treatments
