Article Source: What you need to know about the COVID Vaccine – Everly Report 2021
In this article we will get straight to the point and go over the details provided by the manufacturer (specifically Pfizer, with sources for information on the Moderna vaccine at the end as well) based on the vaccine package insert, published phase 3 clinical trial, and FDA Briefing document.
We will cover ingredients, effectiveness and how it was evaluated, and safety, including reports of adverse events since administration of the vaccine began in December.
Pfizer-BioNTech COVID Vaccine
INGREDIENTS:
“The Pfizer-BioNTech COVID-19 Vaccine is supplied as a frozen suspension in multiple dose vials; each vial must be diluted with 1.8 mL of sterile 0.9% Sodium Chloride Injection, USP prior to use to form the vaccine. Each dose of the Pfizer-BioNTech COVID-19 Vaccine contains 30 mcg of a nucleoside-modified messenger RNA (modRNA) encoding the viral spike (S) glycoprotein of SARS-CoV-2.
Each dose of the Pfizer-BioNTech COVID-19 Vaccine also includes the following ingredients: lipids (0.43 mg (4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 0.05 mg 2[(polyethylene glycol)-2000]- N,N-ditetradecylacetamide, 0.09 mg 1,2-distearoyl-sn-glycero-3-phosphocholine, and 0.2 mg cholesterol), 0.01 mg potassium chloride, 0.01 mg monobasic potassium phosphate, 0.36 mg sodium chloride, 0.07 mg dibasic sodium phosphate dihydrate, and 6 mg sucrose. The diluent (0.9% Sodium Chloride Injection, USP) contributes an additional 2.16 mg sodium chloride per dose.”
Source: Pfizer mRNA Vaccine Insert. (Page 26) https://labeling.pfizer.com/ShowLabeling.aspx
Ingredient of concern: Modified mRNA.
COVID vaccines are the first vaccine to use modified (synthetic) mRNA technology. There is ongoing debate and concern amongst the scientific and medical community with regard to potential unknown effects of injecting lab-created genetic material into the body.
“…there are unique and unknown risks to messenger RNA vaccines, including local and systemic inflammatory responses that could lead to autoimmune conditions.
An article published by the National Center for Biotechnology Information, a division of the National Institutes of Health, said other risks include the bio-distribution and persistence of the induced immunogen expression; possible development of auto-reactive antibodies; and toxic effects of any non-native nucleotides and delivery system components.”
Source: Could mRNA COVID-19 vaccines be dangerous in the long-term? https://m.jpost.com/health-science/could-an-mrna-vaccine-be-dangerous-in-the-long-term-649253
Typically, when mRNA is present outside of a cell, it degrades relatively quickly. In order to prevent this from happening, scientists encapsulated the mRNA in a lipid nanoparticle. How long the mRNA remains in the body to continue being translated into the viral spike protein, is unknown.
Ingredient of concern: Polyethylene Glycol (PEG).
Polyethylene glycol is one of the ingredients used for the lipid nanoparticle that protects the mRNA sequence. Traditional vaccines often contain a chemical substance like aluminum which provokes the immune system to attack the contents of the vaccine. Although mRNA vaccines do not contain aluminum or other traditional adjuvants, the proprietary PEGylated lipid nanoparticle designed by Pfizer is said to have “adjuvant activity”.
Additionally… “[Polyethylene glycol, or PEG] has never been used before in an approved vaccine, but it is found in many drugs that have occasionally triggered anaphylaxis—a potentially life-threatening reaction that can cause rashes, a plummeting blood pressure, shortness of breath, and a fast heartbeat. Some allergists and immunologists believe a small number of people previously exposed to PEG may have high levels of antibodies against PEG, putting them at risk of an anaphylactic reaction to the vaccine.”
EFFECTIVENESS:
Claim: 95% effective
What this claim is based on:
Although there were a total of 43,548 trial participants in Pfizer’s phase 3 trial, their calculation of effectiveness is based on a total of 170 participants.
These 170 participants were the first to develop symptoms of COVID and test positive for SARS-CoV-2, within the two-month monitoring period starting 7 days after the administration of the second vaccine.
From the chart below, it states that of the first 170 participants to develop symptoms and test positive for SARS-CoV-2, 162 of them were placebo recipients and 8 were vaccine recipients.
This is where the “95% effective” calculation comes from.
Source: Pfizer Vaccine Insert.
However, the rest of the participants were not tested for infection, nor were they tested for the development of antibodies, which is the endpoint typically used to measure vaccine effectiveness.
The published phase 3 clinical trial states the following “Limitations and Remaining Questions”:
Note: Remaining questions include “whether the vaccine protects against asymptomatic infection and transmission”.
Source: Phase 3 Clinical Trial. https://www.nejm.org/doi/full/10.1056/NEJMoa2034577
Pfizer’s phase three trial was designed to determine if the vaccine may prevent (or suppress) symptoms, but not infection. Therefore, it is not known whether or not the vaccine actually prevents (asymptomatic) infection or transmission.
Why is this important?
First, the ability of a vaccine to suppress symptoms of infection, but not prevent infection itself, is not an indication or measure of immunity. If an individual is infected with a virus while displaying no symptoms of that infection, that makes them an asymptomatic carrier. Immunity develops with an immune system response. Typically, symptoms are the sign of that immune system response.
Second, although it wasn’t initially studied, the FDA discovered several decades after current pertussis vaccines were put into use, that the pertussis vaccine does not prevent infection or transmission and that vaccinated asymptomatic carriers have actually contributed to pertussis outbreaks.
Third, if the COVID vaccine does not prevent infection or transmission, and only suppresses symptoms, then herd immunity is not possible with this vaccine, even if 100% of the population receives it.
This is precisely why Dr. Fauci and others have stated that regardless of whether or not you receive the vaccines, you will still be expected to wear masks, social distance, avoid gatherings, etc. https://www.npr.org/sections/health-shots/2021/01/12/956051995/why-you-should-still-wear-a-mask-and-avoid-crowds-after-getting-the-covid-19-vac
But let’s go back to the “95% effective” claim.
To add more clarity to previous statements, participants who tested positive for or developed symptoms of COVID infection within the first four weeks after the first shot, were not included in the analysis on effectiveness.
The vaccine insert states that the population assessed for the primary efficacy endpoint (preventing symptoms) includes:
“All eligible randomized participants who receive all vaccination(s) … and have no evidence of SARS-CoV-2 infection prior to 7 days after Dose 2.”
What do we know about the risk of COVID symptoms or infection during the initial four weeks after the first shot?
From the FDA Briefing document below, Pfizer shares the following regarding “Suspected COVID-19 Cases” on page 42:
“Suspected COVID-19 cases that occurred within 7 days after any vaccination were 409 in the vaccine group vs. 287 in the placebo group.”
Source: Pfizer’s FDA Briefing document: https://www.fda.gov/media/144245/download
This means that over 70% of the participants who developed symptoms of COVID in the first 7 days following each shot, were those who received the vaccine.
It also states:
“Among 3410 total cases of suspected but unconfirmed COVID-19 in the overall study population, 1594 occurred in the vaccine group vs. 1816 in the placebo group.”
Therefore, over the entire duration of the trial (about 105 days), almost half (47%) of the participants who developed symptoms identical to COVID, received the vaccine (vs 53% of placebo recipients).
While it was stated previously, let’s reiterate what exactly the vaccine prevents:
“The protocol-specified 2-dose vaccination regimen was [95%] effective in preventing PCR- confirmed COVID-19 occurring at least 7 days after completion of the vaccination regimen.”
(Does not include suspected but untested cases of COVID during the length of the trial, nor confirmed cases of COVID in the first four weeks after the first shot.)
Unfortunately, even the PCR test (whether it is positive or negative) is subject to interpretation, as stated by PCR test manufacturers and the World Health Organization. Pfizer does not state how many cycles was used to determine positivity of participant tests. Therefore it is difficult to know how reliable their testing method actually was.
More about the PCR test, here: https://everlyreport.com/world-health-organization-pcr-test-recommendation/
And yes, you can still contract COVID and test positive for the virus even after receiving both doses of the vaccine.
Congressman tests positive for COVID virus a few weeks after receiving second dose of Pfizer vaccine. https://www.foxnews.com/us/congressman-second-covid-19-dose-tests-positive-virus
Does the vaccine at least prevent death from COVID?
Regarding the ability of the vaccine to reduce mortality from COVID, the FDA briefing document states:
“A larger number of individuals at high risk of COVID-19 and higher attack rates would be needed to confirm efficacy of the vaccine against mortality.”
Mortality was not evaluated because there were no deaths from COVID in their trial, even amongst 21,728 participants who received placebo.
SAFETY:
Safety was evaluated for a “median of two months” during the phase 3 Pfizer clinical trial.
“The most commonly reported systemic events were fatigue and headache (59% and 52%, respectively)…”
“Fever (temperature, ≥38°C) was reported after the second dose by 16% of younger vaccine recipients and by 11% of older recipients.”
“More [vaccine] recipients than placebo recipients reported any adverse event (27% and 12%, respectively) or a related adverse event (21% and 5%).”
Ultimately, Pfizer suggests that the amount and severity of adverse events from the vaccine compared to the placebo are not significant enough.
However, if the vaccine is intended to prevent symptoms (and whether it’s actually 95% effective at doing so is up to you to decide), while also causing significantly more symptoms than placebo (over twice as much), what is the true net benefit? How will we know if there will be a net benefit, in the end?
Although Pfizer states in their published trial that “safety monitoring will continue for 2 years after administration of the second dose of vaccine”, participants in the trial who received placebo are already being offered the vaccine.
Therefore, there will be no placebo group left to compare chronic adverse events, to see whether or not more long term, long lasting health conditions such as autoimmunity, neurological disease, or cancer, are more or less prevalent in the vaccine group compared to placebo.
Therefore, we won’t be receiving much more scientifically-founded information with regard to safety of the vaccine beyond a few months, from the ongoing trial itself.
Opinion: The loss of a control group moving forward and the lack of long term safety data monitoring should be of great concern to everyone, since these are the first mRNA vaccines being distributed to the public, and this technology itself is still experimental. That the Pfizer and Moderna vaccines have not obtained full FDA review and approval, and have only been accepted under Emergency Use Authorization.
From the previously mentioned article on the potential for mRNA vaccines to be dangerous long term:
“We will have a safety profile for only a certain number of months, so if there is a long-term effect after two years, we cannot know,” Brosh said, adding that we could wait two years to discover them, “but then we would have the coronavirus for two more years.”
Linial expressed similar sentiments: “Classical vaccines were designed to take 10 years to develop.”
Pfizer did it in about 9 months.
What else can we conclude about safety from what we know about the trial?
It’s important to understand that the reported adverse events from the trial did not capture or reflect all possible adverse events that will occur when it is administered to the public.
The US population is around 330 million people. There were 21,720 participants who received the vaccine in Pfizer’s clinical trial. In a sense, every 1 participant in the trial represents 15,000 other people in the US who could potentially receive the vaccine. Yet, the demographics of the trial participants do not reflect the demographics of the US population, based on health, age, ethnicity, chronic conditions, etc. (Page 20 of the FDA briefing document.)
“I think people need to understand that the issue of the safety goes well beyond the confines of a clinical trial,” Fauci told CNBC.
“Because when you’re in a clinical trial, you’re giving it for example, the Pfizer trial was 44,000 people. Once you decide to dispense the vaccine widely, you’re talking about millions and tens of millions and ultimately hundreds of millions of doses. So you may see reactions that you didn’t see in the clinical trial,” added Fauci, who is director of the National Institute of Allergy and Infectious Diseases.
Here’s a bit of an example why rare adverse events in clinical trials are important to consider:
From the FDA briefing document on page 33, it states that the difference between the number of Serious Adverse Events (SAEs) between the placebo and the vaccine group is just 0.1%. The placebo group had an SAE rate of 0.5% and vaccine group had an SAE rate of 0.6%. If the background rate of serious adverse events in our population is equivalent to placebo, then the added rate of serious adverse events from the vaccine would be 0.1%.
What is 0.1% of 330 million people?
330,000 people.
And that reflects only the serious adverse events that they were able to capture in the clinical trial, based on a demographic group that is generally healthier than what is representative of the US population as a whole.
What about reported adverse events since the vaccine began to be administered and distributed?
Adverse events are supposed to be reported to VAERS, the Vaccine Adverse Events Reporting System, under the Department of Health and Human Services (HHS). Unfortunately, an HHS study found that less than 1% of vaccine adverse events are actually reported to VAERS.
In addition, the VAERS system search tool and downloadable data in order to access and read VAERS reports are difficult to use for the average person. If you would like, you may attempt to use it yourself, here: https://wonder.cdc.gov/vaers.html
Here is an example of search results from January 29th, 2021 for COVID vaccines:
Thankfully, an independent group of individuals who are concerned with transparency have created a website and search tool of their own which uses VAERS’s raw data. If you would like to explore this system, you may do so here.
Otherwise, with regard to safety, on December 19th, 2020, the CDC presented data regarding adverse events reported during the first 5 days vaccines were administered, December 14th-18th.
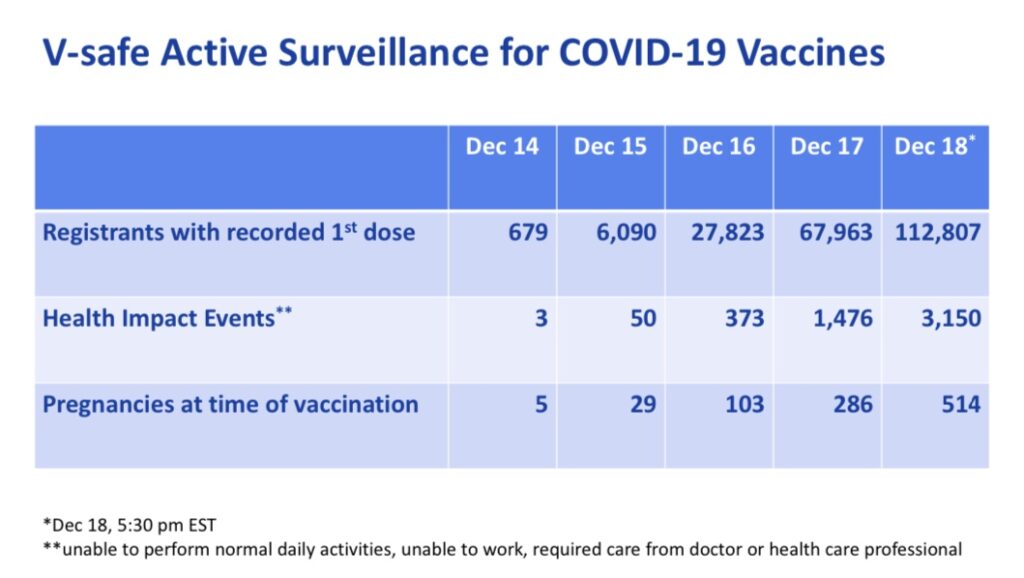
According to the data received, 2.8% of COVID vaccine recipients experienced “Health Impact Events” which made them unable to perform normal daily activities, unable to work, and/or required care from a doctor or health care professional.
This is a relatively high percentage of significant adverse events considering the amount of people who intend to receive the vaccine, and this is just for the first dose. The second dose is known to be more reactogenic for more people (often causing more adverse events).
There have also been several reports of deaths of health care workers, elderly patients, and others shortly after receiving the COVID vaccine (the following list does not encompass all media reports):
Portuguese health worker, 41, dies two days after getting the Pfizer covid vaccine as her father says he ‘wants answers’. https://www.dailymail.co.uk/news/article-9111311/Portuguese-health-worker-41-dies-two-days-getting-Pfizer-covid-vaccine.html
Doctor’s Death After Covid Vaccine Is Being Investigated. https://www.google.com/amp/s/www.nytimes.com/2021/01/12/health/covid-vaccine-death.amp.html
75-year-old man dies of heart attack shortly after receiving coronavirus vaccine. https://www.i24news.tv/en/news/coronavirus/1609155691-israel-75-year-old-man-dies-of-heart-attack-shortly-after-receiving-coronavirus-vaccine
Health care worker dies after second dose of COVID vaccine, investigations underway. https://www.ocregister.com/2021/01/26/health-care-worker-dies-after-second-dose-of-covid-vaccine-investigations-underway/#
Person in Placer County dies shortly after being given COVID-19 vaccine, authorities say. https://www.sacbee.com/news/coronavirus/article248731805.html#storylink=cpy
23 die in Norway after receiving Pfizer COVID-19 vaccine: officials. https://nypost.com/2021/01/15/23-die-in-norway-after-receiving-pfizer-covid-19-vaccine/
South Dakota reports 2 deaths recorded after COVID-19 vaccinations. https://www.blackhillsfox.com/2021/01/20/south-dakota-reports-2-deaths-recorded-after-covid-19-vaccinations/
Auburn Woman Warns She Saw Grandfather’s Aid Die After COVID Vaccine. https://sacramento.cbslocal.com/2021/01/25/no-link-found-yet-auburn-woman-warns-she-saw-grandfathers-aid-die-after-covid-vaccine/
Dozens of care home residents died with Covid before second jab. https://metro.co.uk/2021/01/24/dozens-of-care-home-residents-died-with-covid-after-first-jab-13956611 (After the first jab.)
Family, community mourn loss of Provo NICU therapist to COVID-19*. https://www.fox13now.com/news/coronavirus/local-coronavirus-news/family-community-mourn-loss-of-provo-nicu-therapist-to-covid-19 (*The NICU therapist had received the COVID vaccine 6 days before testing positive for the virus and eventually passing.)
Of course, the deaths occurring in the articles above are not stated as considered to be caused by the vaccine.
Yet in some cases, it is possible that the vaccine even contributed to enhanced COVID disease, due to a phenomenon known as antibody dependent enhancement. In prior vaccine trials which were abandoned, it was discovered that the development of antibodies in response to the experimental vaccine actually made symptoms of disease more severe when the subject was challenged with the virus.
The FDA also considers “vaccine enhanced disease” a possibility when planning for the collection of safety data on COVID vaccine adverse events.
Side note: While most think or believe that autopsies are comprehensive and will find evidence of vaccine injury if it is present in the deceased, it is important to know that there is no protocol in place to determine whether or not a vaccine caused the death of a person, during an autopsy. Unfortunately, parents have had to spend thousands of dollars to order their own private autopsies to begin to look for evidence of vaccine injury when their children die shortly after receiving vaccines.
From a personal contact of mine, Joy Fritz:
“I worked in the mortuary industry doing death certificates for over six years. Vaccine deaths will be chalked up to natural causes. We can never get accurate prevalence rates of how many people die from vaccines, medications or any medical interventions due to cause of death reporting being biased towards natural causes. Death recording protocols are not conducive to scientific investigation of what truly caused a death. Any underlying natural condition that a patient has, no matter how well managed is the default cause of death. The death recording system is not set up to accurately inform public health beliefs, public policy creation or medical decision-making.”
Vaccine resistance by health care professionals
In some areas, 50% of health care workers and up to 60% of nursing home workers are refusing the COVID vaccine. These have been the first individuals to receive the vaccine as it has been rolled out.
Why are they refusing, and are they seeing something that we aren’t seeing?
Large Numbers Of Health Care And Frontline Workers Are Refusing Covid-19 Vaccine. https://www.google.com/amp/s/www.forbes.com/sites/tommybeer/2021/01/02/large-numbers-of-health-care-and-frontline-workers-are-refusing-covid-19-vaccine/amp/
Criminal convictions
I think it’s important for people to know that while developing vaccines may seem like a product of good will, unfortunately the pharmaceutical industry has a long history of criminal convictions for a wide variety of fraudulent actions, Pfizer included. In 2009, Pfizer was ordered to pay $2.3 Billion for fraud they committed.
“Pfizer has been a ‘habitual offender,’ persistently engaging in illegal and corrupt marketing practices, bribing physicians and suppressing adverse trial results.”
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2875889/
Unfortunately, under the United States Prep Act, Pfizer and other COVID vaccine manufacturers were granted liability immunity from adverse events resulting from the administration of their vaccines. If you are harmed by a COVID vaccine, you cannot sue the manufacturer.
Ultimately, I do not seek to make conclusions about the information presented above, nor do I want to provide much personal opinion on the matter. The decision to receive any vaccine is yours and yours alone. However I hope that the above information helps you to be better informed and better equipped for that decision.
Thank you for reading and sharing.
Additional information below.
Human Aborted Fetal Tissue
I wanted to include a note on the use of human aborted fetal cell lines in the production, development, and testing of coronavirus vaccines.
Both Pfizer and Moderna used human aborted fetal cell lines in the testing of their COVID vaccines. The following chart is compiled by the Sound Choice Pharmaceutical Institute.
https://soundchoice.org/vaccines/covid-19-vaccine-chart/
Sound Choice is led by Dr. Theresa Deisher, PhD who has over 30 years of pharmaceutical research and discovered adult cardiac derived stem cells. She has been working on the therapeutic use of adult stem cells as an alternative to aborted fetal cells.
More about the use of aborted fetal cells in COVID vaccine production, here.
Moderna
Vaccine insert: https://www.modernatx.com/covid19vaccine-eua/eua-fact-sheet-providers.pdf
Phase 3 Clinical Trial: https://www.nejm.org/doi/full/10.1056/NEJMoa2035389
FDA Briefing Document: https://www.fda.gov/media/144434/download Pg. 42 Serious Adverse Events “SAEs”.
