KEY POINTS:
– The Johnson & Johnson vector-based vaccine is made using genetically engineered adenovirus and genetically modified human fetal cells.
– Previous attempts at adenovirus-vector vaccines have been abandoned 3 and 4 years into clinical trials.
– The calculated effectiveness of the vaccine to prevent moderate to severe COVID in high risk groups is low.
– One-third of vaccine recipients aged 60 or over experienced “Grade 3” pain which prompted the use of prescription pain killers or prevented daily activities. Nearly half experienced systemic effects (headache, nausea/vomiting, myalgia, fever, etc.).
– An increased risk of blood clots may be a concern with this vaccine.
– Johnson & Johnson has a long history of criminal convictions, repeatedly for knowingly putting the public at risk of harm from their products.
Johnson & Johnson / Janssen COVID Vaccine
INGREDIENTS:
“The vaccine consists of a replication-incompetent recombinant adenovirus type 26 (Ad26) vector expressing the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike (S) protein in a stabilized conformation.
The Ad26 vector expressing the SARS-CoV-2 S protein is grown in PER.C6 TetR cells, in media containing amino acids and no animal-derived proteins.
Each 0.5 mL dose of Janssen COVID-19 Vaccine is formulated to contain 5×1010 virus particles (VP) and the following inactive ingredients: citric acid monohydrate (0.14 mg), trisodium citrate dihydrate (2.02 mg), ethanol (2.04 mg), 2-hydroxypropyl-β-cyclodextrin (HBCD) (25.50 mg), polysorbate-80 (0.16 mg), sodium chloride (2.19 mg). Each dose may also contain residual amounts of host cell proteins (≤0.15 mcg) and/or host cell DNA (≤3 ng).”
Source: Janssen COVID Vaccine Insert, page 17 & 18. https://www.janssenlabels.com/emergency-use-authorization/Janssen+COVID-19+Vaccine-HCP-fact-sheet.pdf
Ingredient of concern: Genetically engineered human adenovirus.
Vector-based COVID vaccines use live viruses which have been genetically modified to remove genes for replication, and to carry and express the SARS-CoV-2 spike protein gene sequence.
“Janssen’s AdVac® vectors are based on a specific type of adenovirus. This type of adenovirus has been genetically modified so that it can no longer multiply in humans and cannot cause disease.”
“AdVac® technology works as follows: an adenovirus [causes common cold] is used as a vector (a carrier) of the genetic code of an antigen [or virus]. Antigens are substances foreign to the human body that trigger an immune response.”
Source: Janssen website. https://www.janssen.com/belgium/janssen-vaccine-technology
“Most often to be used as a vaccine vector, adenoviruses are rendered replication-deficient, meaning they cannot reproduce in vaccine recipients. This is accomplished by removing two genes that the adenovirus needs to reproduce and replacing it with the gene for the protein of interest — in this case the SARS-CoV-2 spike protein.
Source: Children’s Hospital of Philadelphia (CHOP). https://www.chop.edu/news/news-views-getting-familiar-covid-19-adenovirus-replication-deficient-vaccines
Authors of a study published in 2017 reviewed the use of adenoviruses as vectors for gene therapy and vaccines:
“In the early 2000s, Merck began manufacturing a mixed-valent vaccine product composed of three [adenovirus]-based vectors expressing several HIV-1 proteins. This product was evaluated in the notable STEP study, which began enrolling volunteer subjects from around the world in 2004.”
“[T]he study was prematurely stopped in 2007 [three years later] when subjects in the treatment group revealed higher rates of HIV infection than those in the placebo group.”
“Though multiple theories have surfaced to explain the increased trend for HIV acquisition in this treatment arm… none such theories have undergone direct investigation, and so this phenomenon remains to be completely understood.”
“An NIH-sponsored phase II clinical trial (called the “HVTN trial”) began in 2009 and exposed subjects first to a DNA-based primer vaccine expressing HIV proteins to then be followed by a booster [adenovirus] vector vaccine expressing the same proteins. Yet again, this trial was brought to an early end in 2013 [four years later] when the vaccine failed to reduce the rate of HIV infection or attenuate disease contracted by subjects during the trial.”
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5609467/
From the American Chemical Society:
“J&J calls its adenoviral vector platform a ‘proven’ technology.
While adenoviral vectors have been tested in far more people than mRNA vaccines, the technology is used in only one commercial vaccine today: a rabies vaccine used to immunize wild animals. So far, no adenoviral vector vaccines have demonstrated they can prevent disease in humans.”
‘It is not proven until it is licensed, and [if] in postlicensure, continues to succeed,’ Ertl says. ‘To say it is proven without peer-reviewed efficacy data is a stretch.’”
Ingredient of concern: PER.c6 cells (Primary embryonic retinal cells.)
“Because viruses need cells to reproduce and the [genetically modified] virus cannot reproduce, scientists need a way to make large volumes of the altered vaccine virus. This is most often accomplished using cell lines [e.g. PER.c6] that have been modified to include the gene that was removed from the adenovirus.”
Source: Children’s Hospital of Philadelphia (CHOP). https://www.chop.edu/news/news-views-getting-familiar-covid-19-adenovirus-replication-deficient-vaccines
“Cultured human cell lines (e.g., PER.c6, HEK293) are used like ‘factories’ to produce large quantities of the engineered viruses.”
Source: https://lozierinstitute.org/a-visual-aid-to-viral-infection-and-vaccine-production/
PER.c6 cells were harvested from the retina (eyes) of a human embryo (fetus). Residual fragments of DNA from these cells remains in the vaccines administered (see ingredients, above).
“PER.C6 cells were originally designed as helper cells to package adenoviral vectors without generating replication competent adenovirus (RCA).”
“PER.C6 cells are immortalized primary human embryonic retinal cells engineered to express [specific genes].”
Source: https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(02)00045-X
“[T]his cell line, originally isolated in 1985, was developed from human embryonic retinal cells in the late 1990s. While these cells are less likely to restore the ability [of the adenovirus] to replicate, this can still occur on occasion…” [emphasis mine]
Source: CHOP. https://www.chop.edu/news/news-views-getting-familiar-covid-19-adenovirus-replication-deficient-vaccines
Ingredient of concern: Polysorbate 80
Polysorbate 80 is also known as “tween 80” or “PS80” or “P80”. It’s an emulsifier and excipient used to stabilize the formulation of medical products. It also functions as a surfactant / detergent (disrupts lipid membranes) and is used in a wide variety of health and beauty products, and processed foods.
Research has shown that polysorbate 80 can negatively affect the gut microbiome and increase anxiety when consumed, and some individuals may be additionally sensitive to injecting polysorbate 80 directly into the body.
Polysorbate 80 can cause severe anaphylactic reactions:
“Conclusion: Polysorbate 80 is a ubiquitously used solubilizing agent that can cause severe nonimmunologic anaphylactoid reactions.”
Source: https://www.sciencedirect.com/science/article/abs/pii/S1081120610610241
Read also: Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. https://pubmed.ncbi.nlm.nih.gov/22605841/
Polysorbate 80 has also been found to be particularly useful for transporting drugs across the typically difficult to penetrate blood brain barrier. This is because polysorbate disrupts lipid membranes.
“The non-ionic surfactant, polysorbate 80 as a coating material promises an unparalleled opportunity for enhancement of brain targeting of colloidal particles.”
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC539329/
EFFECTIVENESS:
Claim: 66.9% effective
What this claim is based on:
Although there are a total of 43,783 participants in Janssen’s ongoing phase 3 clinical trial, their calculation on effectiveness is based on a total of 464 participants.
These 464 participants were the first to develop “moderate to severe/critical COVID-19” symptoms and test positive for SARS-CoV-2, within the two-month monitoring period starting 14 days after the administration of the vaccine.
Of the first 464 participants to develop moderate to severe COVID symptoms and test positive for SARS-CoV-2, 348 of them were placebo recipients and 116 were vaccine recipients.
To repeat, in the first two months of the trial, 116 vaccine recipients developed moderate to severe COVID symptoms and tested positive for SARS-CoV-2. (This does not include mild cases nor suspected but unconfirmed cases of COVID – this data has not .)
Effectiveness calculation is explained, here.
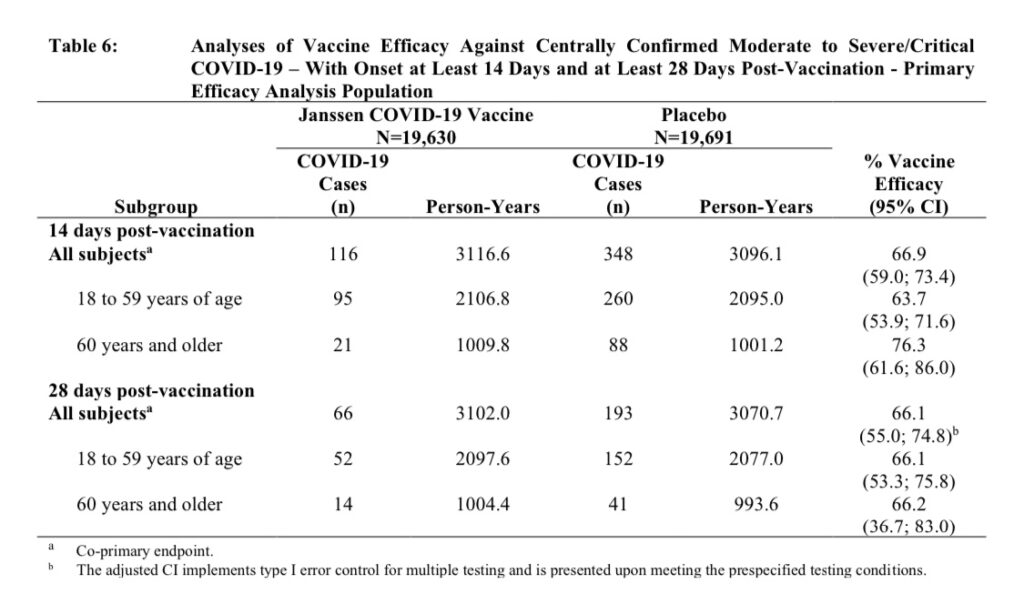
Source: Janssen Vaccine Insert.
Unfortunately, when it comes to protecting those who are most at risk of severe COVID disease – that is, those who are 60 or older and have comorbidities (simultaneous presence of two or more medical conditions or chronic diseases in a patient), the calculated effectiveness of the Janssen vaccine drops to 42.3% – less than a coin toss. (Bottom right in Table 13 of the FDA Briefing Document, below.)
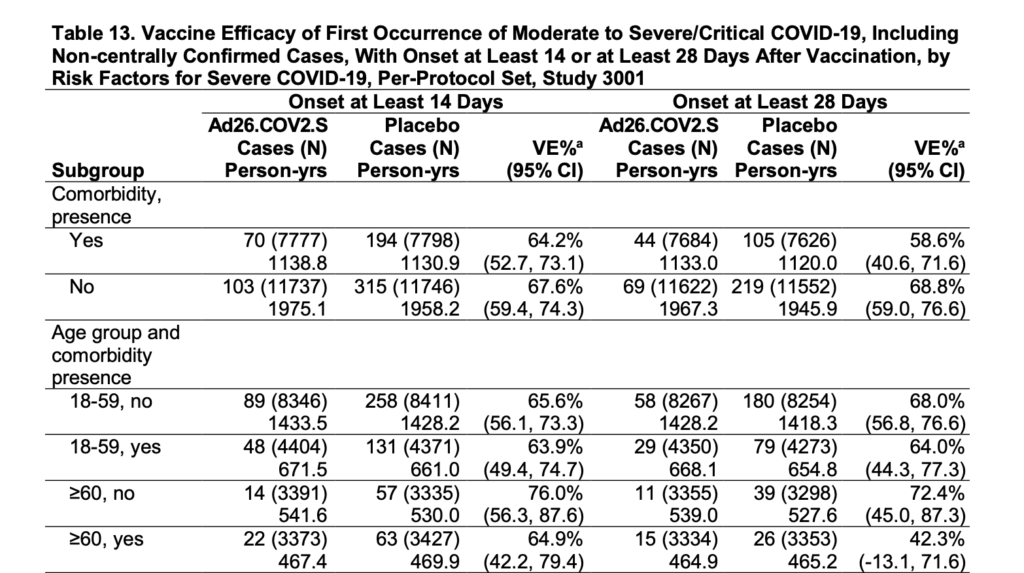
Source: Janssen FDA Briefing Document, page 29.
Note that the confidence interval “(95% CI)” for this value (42.3%) is very wide: -13.1 to 71.6. (This is listed just below “42.3%” on the chart above). What this means is that there is low confidence that the true effectiveness of the vaccine for individuals 60 or over, with comorbidities, is actually 42.3%. It may actually be much, much lower.
A negative value on the low end of the confidence interval (the confidence interval includes a negative value for Vaccine Effectiveness, “VE%”) may indicate that those who received the vaccine in this risk group could be more likely to develop moderate to severe/critical COVID disease, or that there is no significant difference in effectiveness between the vaccine group and placebo.
More on confidence intervals, here, and here.
For those with hypertension or type 2 diabetes, vaccine effectiveness drops to 35.7% and 23%, respectively. The lower bound of the confidence intervals for these values are also negative and reflect that there is low confidence in the effectiveness of the vaccine for these high risk groups.
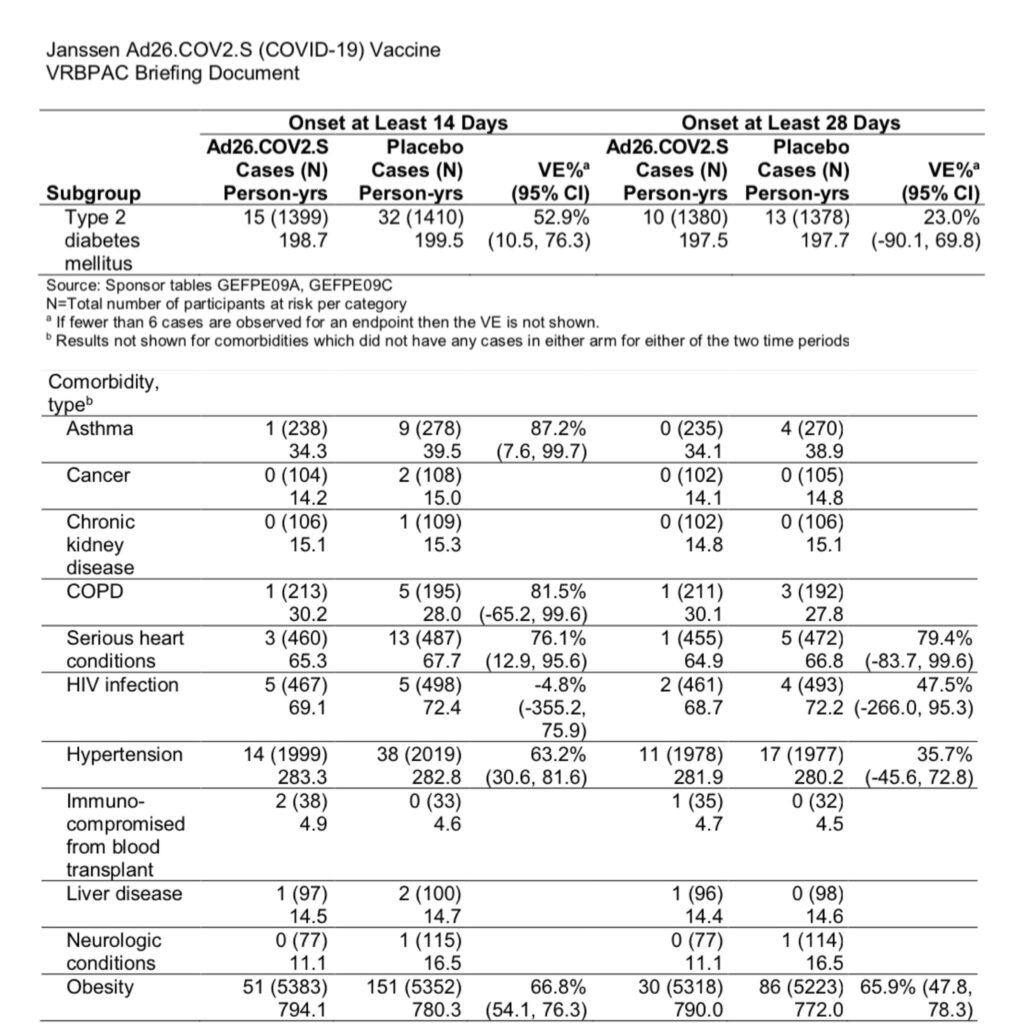
Regarding asymptomatic infection (and therefore potential asymptomatic transmission), Janssen reports that 10 vaccine recipients and 37 placebo recipients seroconverted (initially had no detectable levels of antibodies and tested positive for antibodies after Day 29), without symptoms. Therefore, vaccine recipients are still able to become infected and exhibit no symptoms.
Side note: Janssen admits to removing “possibly symptomatic COVID cases” from the dataset when analyzing asymptomatic infections. If these participants were removed from the dataset for this analysis, it’s unlikely that they were included in the analysis on symptomatic COVID infections and therefore, vaccine effectiveness.
Source: Janssen FDA Briefing Document, page 35 & 36.
Ultimately, it appears from their preliminary data, that the vaccine does not prevent asymptomatic infection. Asymptomatic transmission of the virus from infected vaccine recipients has not been evaluated.
Reminder: Although it wasn’t initially studied, the FDA discovered several decades after current pertussis vaccines were put into use, that the pertussis vaccine does not prevent infection or transmission and that vaccinated asymptomatic carriers have actually contributed to pertussis outbreaks.
If COVID vaccines do not prevent asymptomatic infection or transmission, and only suppress symptoms in some individuals, then herd immunity via vaccination is not possible even if 100% of the population receives it. Herd immunity will only be achieved when a large enough portion of the population is naturally immune.
Does the vaccine prevent death? Media reports claim it is 100% effective at preventing COVID deaths, but what does this mean?
As of the publication of Janssen’s FDA Briefing Document, there were 7 COVID-related deaths reported in their clinical trial. All 7 occurred in the placebo group, and all 7 were at study sites in South Africa.
These 7 cases from South Africa are what is used to claim that the vaccine is “100% effective” against death from COVID.
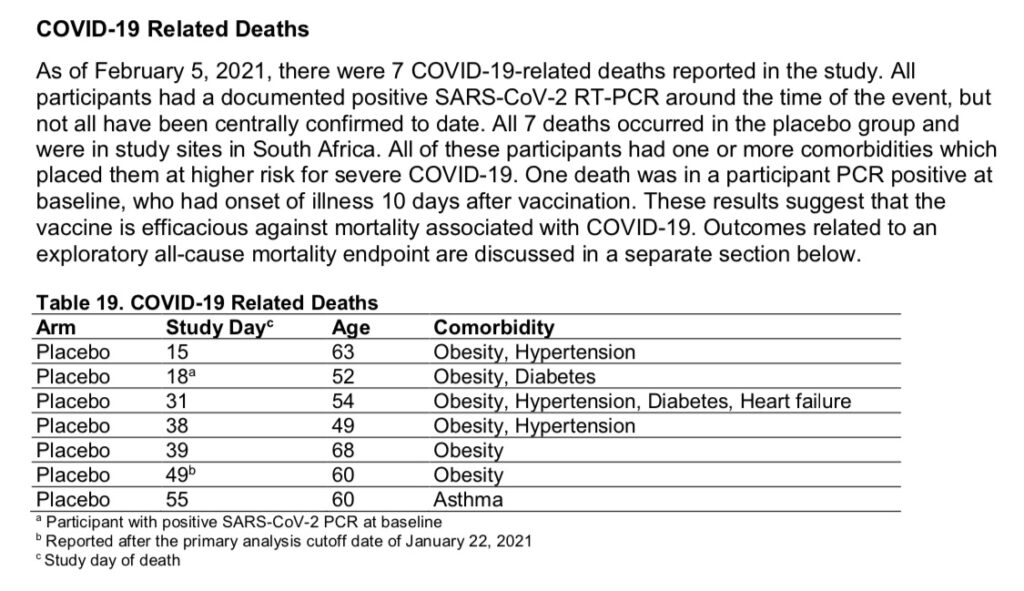
Vaccine effectiveness against all-cause mortality, 28 days post-vaccination, was reported to be 75% (page 36). This is based on 2 deaths occurring in the vaccine group and 8 deaths occurring in the placebo group 28 days post-vaccination. (Percent effectiveness against death dropped after 28 days.)
However, this is out of a total of nearly 22,000 participants in each group, over just 2 months. Therefore, Janssen includes the following:
“There is suggestion of a positive effect on all-cause mortality; however, the confidence interval is wide [-25.2 to 97.4], with a lower bound below 0 after 28 days post-vaccination.”
Again, there is very low confidence in this value, and therefore low confidence that there is a significant difference between the vaccine and placebo.
SAFETY:
Safety has been evaluated for a “median of two months” during the phase 3 Janssen clinical trial. The following chart (table 24 on page 41 of the FDA briefing document) lists the frequency of adverse events at the injection site:
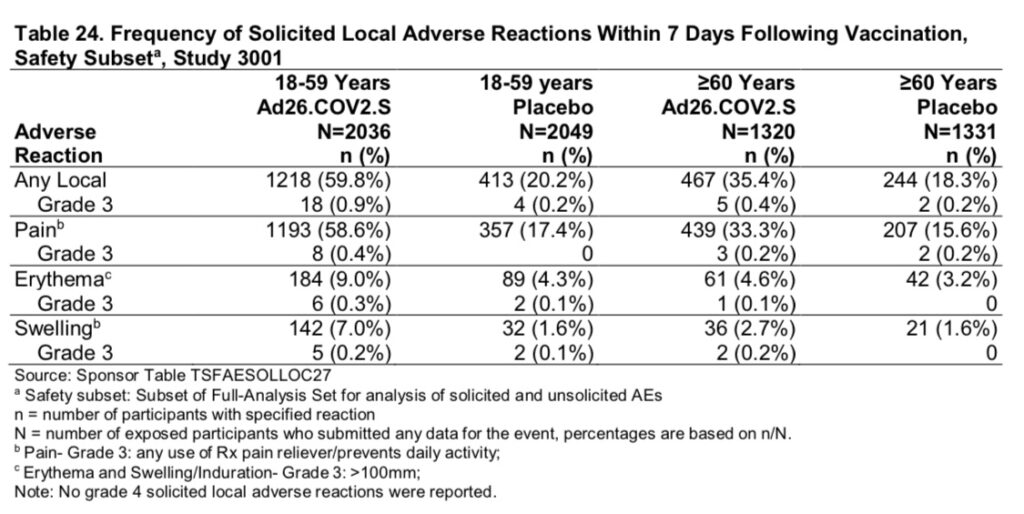
For individuals aged 60 or more, 33.3% of vaccine recipients experienced “Grade 3” pain that prompted use of a prescription pain reliever and/or prevented daily activities, within the first 7 days following the shot.
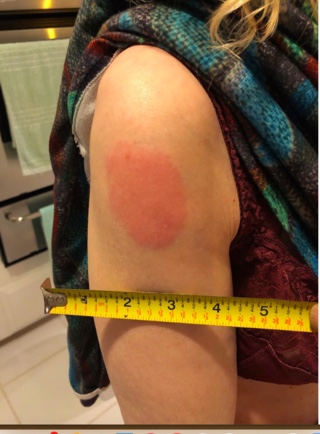
4.6% developed erythema (red skin rash) at the injection site, over 4 inches in size. Photo below for size comparison:
Regarding systemic events, 45.3% of participants aged 60 or over experienced solicited systemic adverse reactions (fatigue, headache, nausea/vomiting, fever, myalgia) within the first 7 days following the vaccine. 7.2% of participants experienced unsolicited systemic adverse reactions which were considered related to the vaccine by the trial investigator.
“Solicited” adverse events are those which are intentionally gathered/requested to be documented by the participant per the study protocol. “Unsolicited” adverse events are those which the participants volunteer to report during the trial.
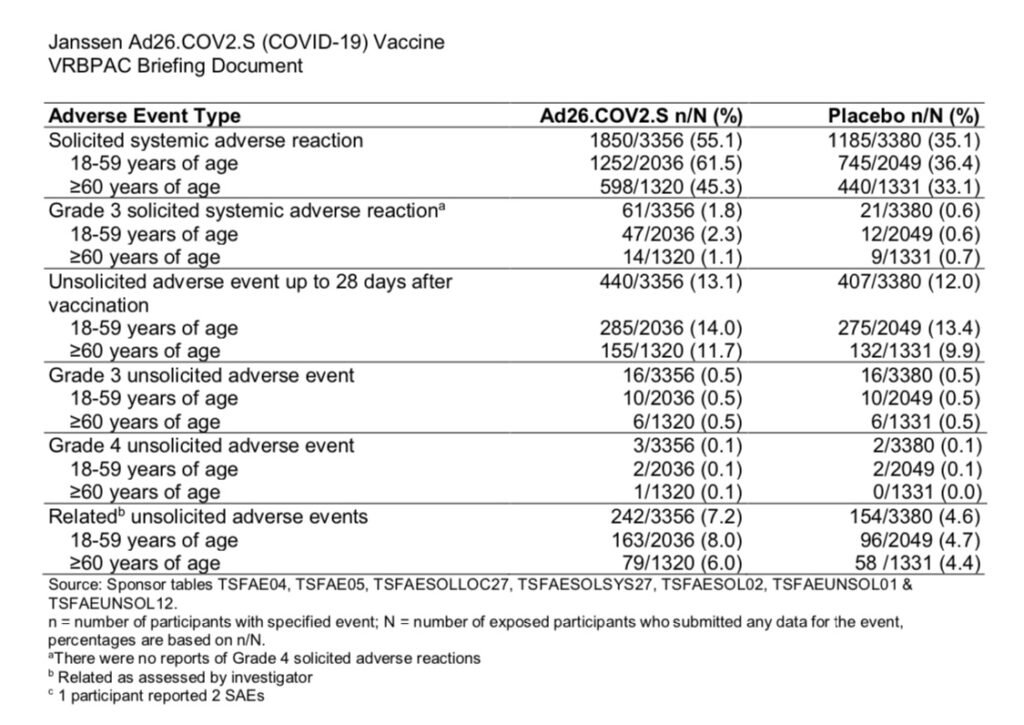
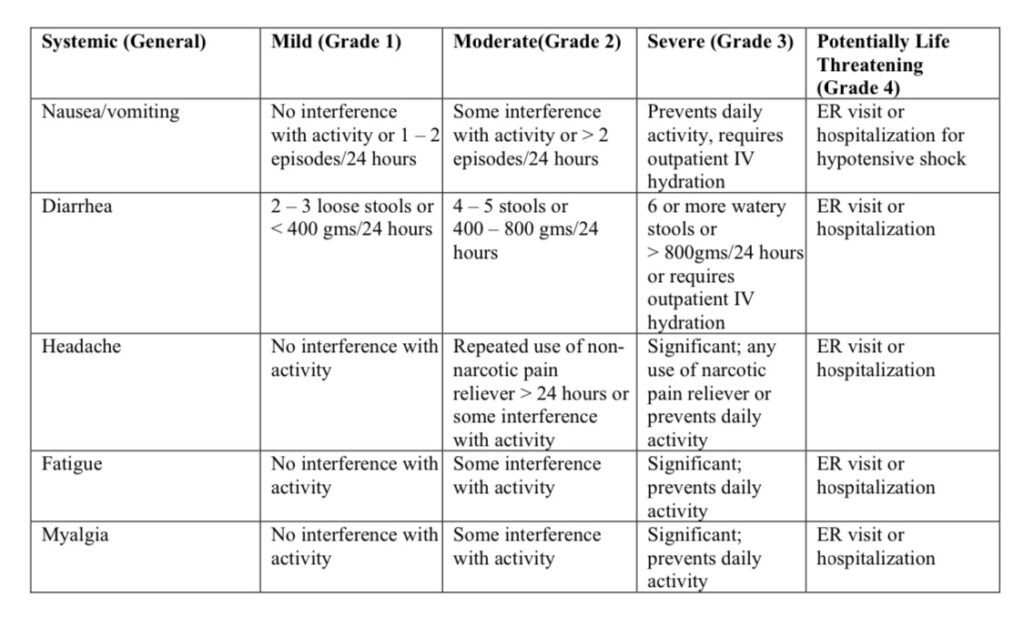
Source: https://www.fda.gov/media/73679/download
Blood clots
One of the serious adverse events of note with regard to the Janssen vaccine were blood clots. 0.06% of vaccine recipients vs 0.05% of placebo recipients experienced thromboembolic events. (Table 29, page 46 & 47 of the FDA Briefing Document.)
If the vaccine causes a 0.01% increase in blood clots, that would mean an additional 500 people out of 5 million receiving the vaccine, would experience a thromboembolic event.
While this may not seem like a large number, AstraZeneca’s vaccine which is also an adenovirus vector-based vaccine, has been under investigation by the European Medicines Agency for causing thromboembolic events, after just 30 cases of blood clots have been reported, out of 5 million vaccinated.
18 countries, including Denmark, Norway, Sweden, Canada, Germany, France, Italy, and Spain, suspended the rollout of the AstraZeneca vaccine because of this serious adverse event.
What about reported adverse events since the vaccine began to be administered and distributed?
Adverse events which occur following vaccination should be reported to VAERS, the Vaccine Adverse Events Reporting System, under the Department of Health and Human Services (HHS). Unfortunately, an HHS study found that less than 1% of adverse events are reported.

In addition, the VAERS system search tool may be difficult to use for the average person: https://wonder.cdc.gov/vaers.html
Currently, the VAERS database is only updated through March 26th, 2021. However, between March 2nd, when the Janssen vaccine began to be administered in the U.S., and March 26th [25 days], there have been 2,790 reports of adverse events submitted to VAERS, including 29 deaths.
Read the 2,790 reports, compiled from the VAERS database, here.
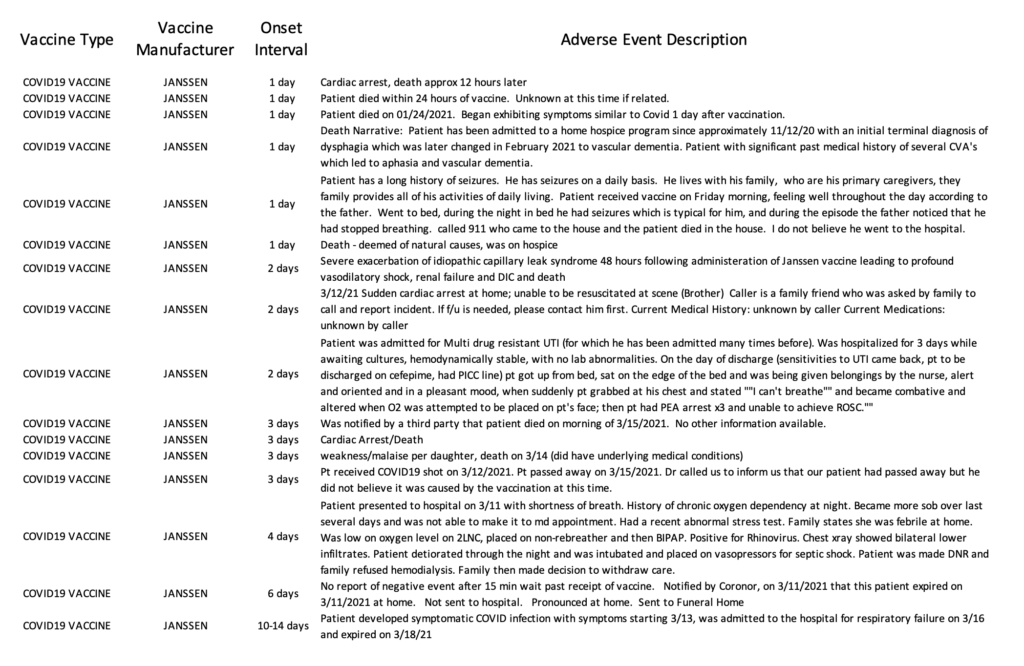
Alternatively, there is an independent group of individuals who are concerned with transparency and usability of the VAERS search tool and have created a website and tool of their own which uses VAERS’s raw data. If you would like to explore this system, you may do so here.
It has been less than a month since the Janssen vaccine began to be rolled out, but adverse events have been reported in the news media, as well:
Man’s skin ‘peeled off’ in rare reaction to Johnson & Johnson COVID vaccine: https://nypost.com/2021/03/30/mans-skin-peeled-off-in-reaction-to-johnson-johnson-covid-vax/
Woman dies of stroke 48 hours after receiving the Johnson & Johnson COVID-19 vaccine: https://www.wral.com/coronavirus/woman-raises-questions-after-mom-s-death-certificate-references-vaccine-experts-warn-about-drawing-conclusions/19594346/
Criminal convictions
I think it’s important for people to know that while developing vaccines may seem like a product of good will, unfortunately the pharmaceutical industry has a long history of criminal convictions for a wide variety of fraudulent actions.
In 2018, Johnson & Johnson came under fire for having known that their baby powder products were contaminated with asbestos.
“[I]nternal documents examined by Reuters show that the company’s powder was sometimes tainted with carcinogenic asbestos and that J&J kept that information from regulators and the public.”
Source: https://www.reuters.com/investigates/special-report/johnsonandjohnson-cancer/
Johnson & Johnson has paid over $4.2 billion in penalties for various crimes since the year 2000, including false claims, drug or medical equipment safety violations, foreign corrupt practices, consumer protection violations, environmental violations, and more.
Of note, in 2013 at a press conference, U.S. Attorney General Eric Holder stated that Johnson & Johnson’s practices “recklessly put at risk the health of some of the most vulnerable members of our society — including young children, the elderly and the disabled.”
Much more, here: https://www.corp-research.org/jnj
And this, just in, on March 31st, 2021:
Biden administration knew more than a week ago of J&J contractor vaccine-supply problems that caused 15 MILLION doses to be halted due to ingredient mix-up as it’s revealed the firm has a string of quality control citations: https://www.dailymail.co.uk/health/article-9424827/15-million-doses-J-J-vaccine-ruined-ingredient-mix-up.html
Unfortunately, under the United States Prep Act, Johnson & Johnson and other COVID vaccine manufacturers were granted liability immunity from adverse events resulting from the administration of their vaccines. If you are harmed by a COVID vaccine, you cannot sue the manufacturer.
Ultimately, I do not seek to make conclusions about the information presented above, nor do I want to provide much personal opinion on the matter. The decision to receive any vaccine is yours and yours alone. However I hope that the above information helps you to be better informed and better equipped for that decision.
Thank you for reading and sharing.
Article Source: What you need to know about Johnson & Johnson’s COVID Vaccine – Everly Report 2021
