As discussed in Part 1 of this series, colonoscopies are a major money-maker for the conventional medicine machine, generating at least $42B per year, year after year. Is it any wonder they backpedal on any suggestions that could reduce the number of annual procedures? The caveat is always ‘colonoscopies are the Gold Standard for screening’ (maybe it’s really just for the GOLD)?The cost of complications are not taken into consideration when analyzing the annual procedure fees.
I also briefly talked about polyps – which (surprisingly) rarely become cancerous. It should be no surprise that doctors can charge more for the procedure if they remove a tiny benign polyp.
In part 2 of this series, we reviewed the risks of colonoscopies, including the REAL number of side effects and complications from this exam, especially when a polyp is removed. We discussed perforations, infections, and the importance of asking your doctor how many scopes s/he has done! The skill of the examiner is an independent risk factor for complications. We also reviewed the new study, published in October, 2022 in New England Journal of Medicine, that calls into doubt the need routine colonoscopies.
A conventional colonoscopy isn’t simply a routine doctor’s visit, but an ambulatory surgical procedure performed under anesthesia. The cancer industry has used its heavy-handed fear and propaganda machine to push tests that are costly, are not benign, may not be necessary and may not even lower the risk of cancer or death.
Here in Part 3,we will review other types of tests available to screen for colon cancer.
- Sigmoidoscopies – do you qualify?
- Testing stool for blood or cancer markers: Guaiac test, FIT test, Cologuard test
- Recent FDA-approved STOOL tests for colon cancer screening
- Various little-known BLOOD tests approved for colon cancer screening
- New tests under development
It makes sense to screen for colon cancer and if present, to identify and remove it at the earliest possible stage. An idealscreening test should be safe, readily available, convenient, inexpensive and have a high sensitivity. Screening colonoscopiesare none of these!
Screening methods for colon cancer are divided into invasive and non-invasive tests.
We’ve already discussed colonoscopy, an invasive test, with all of its risks. Another test, called a flexible sigmoidoscopy (FlexSig) is similar, except it less extensive. A FlexSig is faster (takes about 15 minutes), less complicated, and generally less expensive than colonoscopy. Intravenous sedation is usually not needed, and enemas are used for the preparation instead of the harsh oral preps used for several days prior to a colonoscopy. In addition, a colonoscope is about 66 inches long; a flexible sigmoidoscope is about 24 inches long and has a smaller diameter.
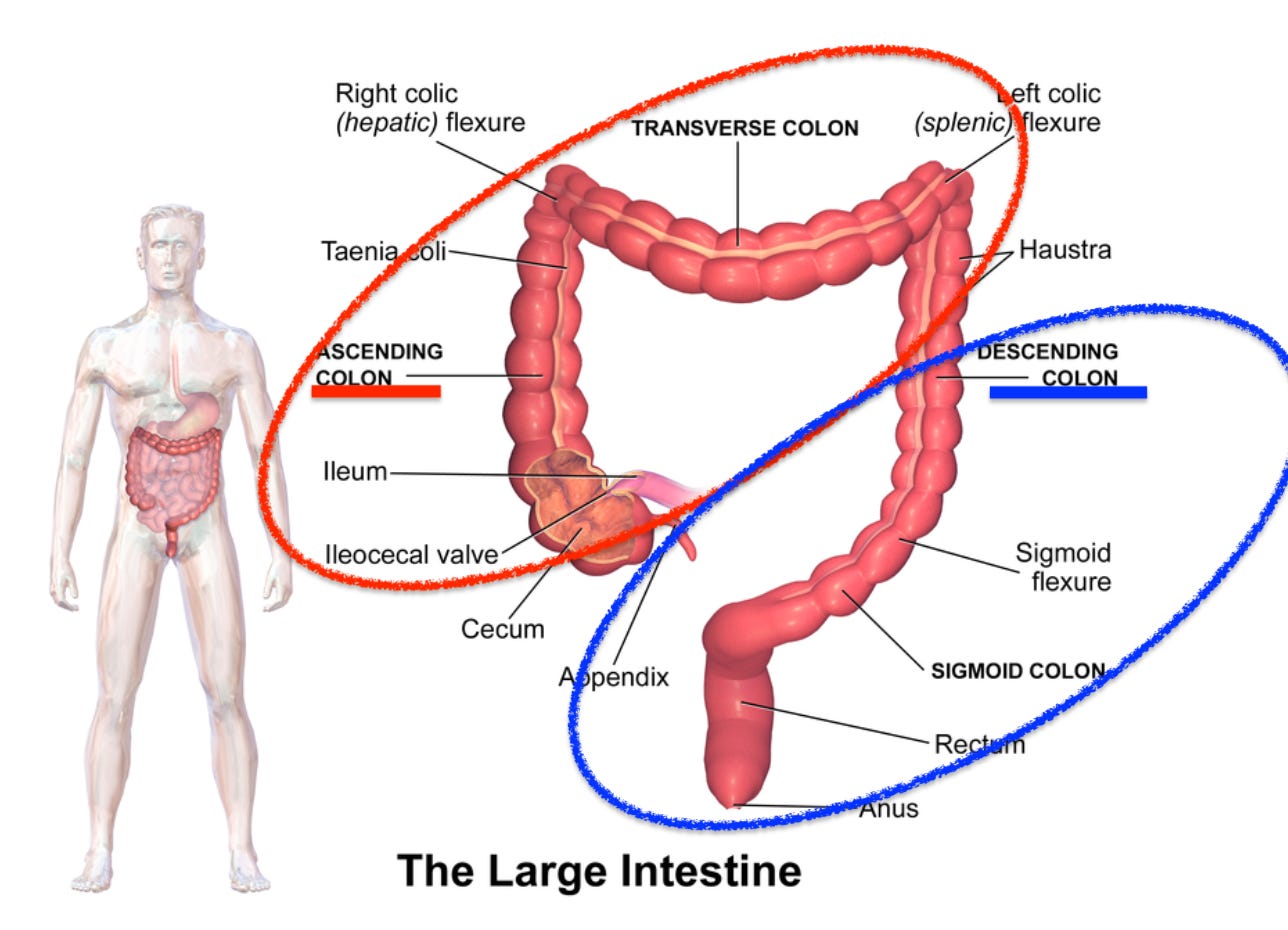
While flexible sigmoidoscopy can usually view the entire descending colon, other areas of the colon go unexamined. Therefore, there is a risk that cancers could go undetected if flexible sigmoidoscopy is undertaken alone.
To sort this out, a study was done in the UK (2018) to determine who can be screened using a FlexSig instead of undergoing a full colonoscopy.’
This is a multicenter study of 7,375 patients with suspected colon cancer who were referred from 21 hospitals throughout England. The study confirmed the strong association betweenanemia and abdominal masswith cancer in the ascending colon (cecum, ascending colon, transverse colon, splenic flexure – see above).
We found that 80% of descending colon cancers could be identified by using a broader definition of anemia [hemoglobin <11 g/dL men; <10 g/dL women]. If a narrower definition of anemia was used [hemoglobin <13 g/dL men; <12 g/dL women], only 39% of cancers were suspected and then identified.
Given the importance of anemia as a marker for colon cancer, we recommend all patients with suspected colon cancer have a full blood count.
This large study confirmed that ascending colon cancers are rare and found in less than 1% of patients without anemia and/or without abdominal mass. Patients who presented with only a change in bowel habits or with only rectal bleeding WITHOUT anemia rarely had colon cancer. Therefore, if the patient is not anemic, s/he is a good candidate for a sigmoidoscopy (FlexSig) for screening every 5 years plus annual fecal blood test instead of undergoing full colonoscopy every 10 years,unless it’s indicated for diagnostic purposes.
Current screening methods for colon cancer are divided into invasive and non-invasive tests. Let’s look at the non-invasive screening tests.
The least invasive and least expensive screening test for colon cancer is to check the stool for the presence of blood. Called a fecal occult blood test. (FOBT), this test has long been the most important ‘first step’ in the screening process for colon cancer. This simple test makes the most sense before immediately recommending colonoscopy for routine ‘screening’.
The guaiac fecal occult blood test (gFOBT) is used to identify blood in the stool that can’t be seen with the naked eye. Abnormal tissues or cancerous lesions are often fragile and can be brushed open as stool passes by, releasing tiny drops of blood.
The test involves placing a tiny amount of fecal matter on card coated with guaiac paper, a type of paper that contains the phenolic compound, alpha-guaiaconic acid, extracted from the wood resin of Guaiacum trees. Next, a small amount of hydrogen peroxide is dropped on the stool. If blood is present, the card yields a blue reaction product within seconds. The test is usually repeated on at least three different samples on different days, especially if the first test is negative because abnormal cells may not bleed all the time. The test should be done on a stool sample that is less than 24 hours old and can be done at home with a kit purchased from the drug store.
Guaiac tests are more sensitive for detecting blood from the lower than from the upper gastrointestinal tract because hemoglobin from red blood cells is degraded as it passes through the gastrointestinal tract. Keep in mind there conditions other than cancer that can release blood into the stool, such as inflammatory bowel disease, an ulcer or hemorrhoids. If this screening test is positive, additional diagnostic tests will be required.
While no prep is required to do the gFOBT, factors have been known to affect the results. It is advisable to avoid these medications and foods for 3 to 5 days before stool testing:
- Anti-inflammatory medications, such as Advil, Aleve, Ibuprofen, and aspirin
- Topical iodine preps
- Vitamin C and citrus fruits
- Red meat
While these avoidance recommendations have long been thought to give a more accurate test, a large Canadian meta-analysis (2010) evaluated this premise and concluded:
Most of the evidence evaluating the effect of dietary restrictions on FOBT results is outdated and of suboptimal quality. However, 4 RCTs and a meta-analysis of these data do not support dietary restrictions when screening for colorectal cancer.
In 2012, the World Endoscopy Organization Expert Working Group recommended FIT as a preferred technology for screening instead of gFOBT.
Similar to the guaiac stool test, the fecal immunochemical test (FIT) is used to identify non-visible blood in the stool. What makes FIT different is that it only detects human blood: it uses an antibody to human globin. There is no cross-reactivity with hemoglobin in dietary meats. Since medication and food do not interfere, the results are more accurate. FIT can accurately identify about 79% of colon cancers. Also, FIT is more specific for identifying blood from the colon. Test kids for home FIT tests are available online or at your local pharmacy.
In August 2014, Cologuard® became the first DNA stool test approved by the FDA for colon cancer screening. Cologuard, manufactured by Exact Sciences, is approved for screening average-risk adults over 50 years old. Cologuard uses molecular tests to identify biomarkers and gene mutations related to colorectal cancer (for those who are research geeks, the markers tested for are KRAS mutation, BMP3, and NDRG4 methylation).
The sensitivity of a one-time screening with Cologuard test is 92% for colorectal cancer, but the sensitivity for advanced adenomas is around 50%. The downside to the Cologuard test is that it’s pricey: it costs between $450 and $700 and it is generally not covered by insurance. But compare that to the out-of-pocket cost of a colonoscopy AND the potential cost of its complications, this test really is a bargain!
An observational study (2017) reported that 97% of subjects refusing colonoscopy accepted a non-invasive screening test. Of these 83% preferred a blood test. Here are two blood tests for colon cancer screening that are generally notsuggested by your doctor!
Septins are a group of scaffolding proteins that provide structural support during cell division.Hypermethylated Septin9 DNAcan be found in the bloodstream as a tumor marker for colon cancer.
Epi proColon is a blood test – which can be obtained through LabCorp – can be used to detect the methylated septin 9 (mSEPT9) gene. Epi proColon is an FDA approved test for colorectal cancer screening. This simple blood test is able to detect a majority of colon cancer at all stages and all colorectal locations. The test should be obtained by individuals of average risk for colon cancer but who are unwilling or unable to undergo colonoscopy or a FlexSig exam.
It has been knownsince 2009 that the mSEPT9 blood test assay can successfully identify 72% of colon cancers with a specificity of 93% (in the training study) and identified 68% of colon cancers with a specificity of 89% (in the testing study.)
The test is reasonably price: Medicare payment rate for the test is $192. Some private insurers consider mSEPT9 to be investigational and may not pay for or reimburse the cost of the test. But again, given the challenges with invasive tests, this is an excellent option for non-invasive screening.
On May 2022, Guardant health announced the availability of Shield™, a blood test for the detection of early-stage colon cancer for asymptomatic individuals, 45yo and older.
The Shield assay demonstrated 91% sensitivity (detection rate) of colon cancer, including 90% for Stage I, 97% for Stage II, and 86% for Stage III colon cancer. The assay also demonstrated 20% sensitivity for advanced adenomas and 92% specificity (true negative rate) in normal cases. The test is not yet widely available, but read about it and ask your doctor to give you this option for screening.
There are multiple modalities under development for alternative screening for colon cancer. I’ve listed a few here for general interest.
Examination of the colon via swallowing a pill, called a capsule endoscopy (CCE), allows minimally invasive assessment of the large bowel. This test has been under development since at least 2009.
Intensive bowel cleansing is required, in similar fashion to colonoscopy. Two meta-analyses of CCE showed this procedure had a sensitivity of 71% to 73% for detection of all polyps and a sensitivity of 68% to 69% for other ‘significant findings.’ While these findings are comparable to other noninvasive measures, the accuracy of the exam is largely dependent on the quality of the colon cleansing prep. The test has been shown to be well tolerated by patients. It is undergoing further research prior to release for general use.
Tumor markers are considered to be one of the best methods for the early detection of asymptomatic micro-focal tumors. Non-coding RNAs (ncRNAs) are a class of endogenous RNAs that are involved in the regulation of gene expression. Circular RNA (circRNA) is a type of ncRNA that can be manipulated into regulating many important biological processes.
circRNA has a high level of stability, making it a potentially valuable tumor marker for many type of cancers, including colon cancer.This research for colon cancer is in its infancy, but tissue biomarkers have long been used to follow treatment progress for several types of cancer.
In the 1980’s and 1990’s, most screening methods put in use were gFOBT and sigmoidoscopy. However, since the year 2000, most colon cancer screening in the United States shifted towards colonoscopy, even though its effects on reducing the incidence and mortality from colon cancer has never been proven in a solid randomized controlled trial.
Therefore, the evident question should be: has the benefit truly been worth the risk? Are there better ways to screen for risks – that then SHOULD have a colonoscopy as a confirmatory study?
Recall, an idealscreening test should be safe, readily available, convenient, inexpensive and have a high sensitivity. I think several stool and blood tests discussed in this substack that are much better for screeningthan an expensive, marginally safe colonoscopy which, in my opinion, should only be used to confirm a diagnosis and should NOT to be used for routine screening.
Studies reviewed, in no particular order:
- 2002:Occult Gastrointestinal Bleeding: Detection, Interpretation, and Evaluation.
- 2009:Circulating methylated SEPT9 DNA as a biomarker for colorectal cancer
- 2011:Septin 9 methylated DNA: sensitive and specific blood test for colon cancer
- 2015:Fecal occult blood testing for colorectal cancer screening: past or future
- 2017: Colorectal cancer screening: An updated review of the available options – IMPORTANT PAPER
- 2018:Colorectal Cancer Screening—Who, How, and When?
- 2019:Fecal occult blood test in colorectal cancer screening
- 2019:Genomic Assessment of Blood-Derived Circulating Tumor DNA in Patients With Colorectal Cancers: Correlation With Tissue Sequencing, Therapeutic Response, and Survival
- 2019:mSEPT9 Blood Test (Epi proColon) for Colorectal Cancer Screening
- 2022:Source matters: a survey of cost variation for fecal immunochemical tests in primary care. (both FOBT and FIT tests cost around $6USD)
- 2022:Systematic analysis of circRNA biomarkers for diagnosis, prognosis and therapy in colorectal cancer
*Source: The Truth About Colonoscopies, Pt 3
