What Safety Studies Have Been Done on mRNA Swine Vaccines?
STORY AT-A-GLANCE
- Unbeknownst to the public, pork producers in the U.S. and Canada have been using customizable mRNA-based “vaccines” on their herds since 2018
- The mRNA platform, Sequivity, is the only part of this gene-based “vaccine” technology that has been approved. All customized mRNA injections created using this platform are untested, and the initial safety testing — upon which the assumed safety of all customized jabs are based — was grossly inadequate
- According to the U.S. Department of Agriculture’s (USDA) summary of studies supporting the product licensure of Sequivity, safety is based on a single study involving 748 piglets, which were given two doses of an unspecified mRNA Sequivity injection
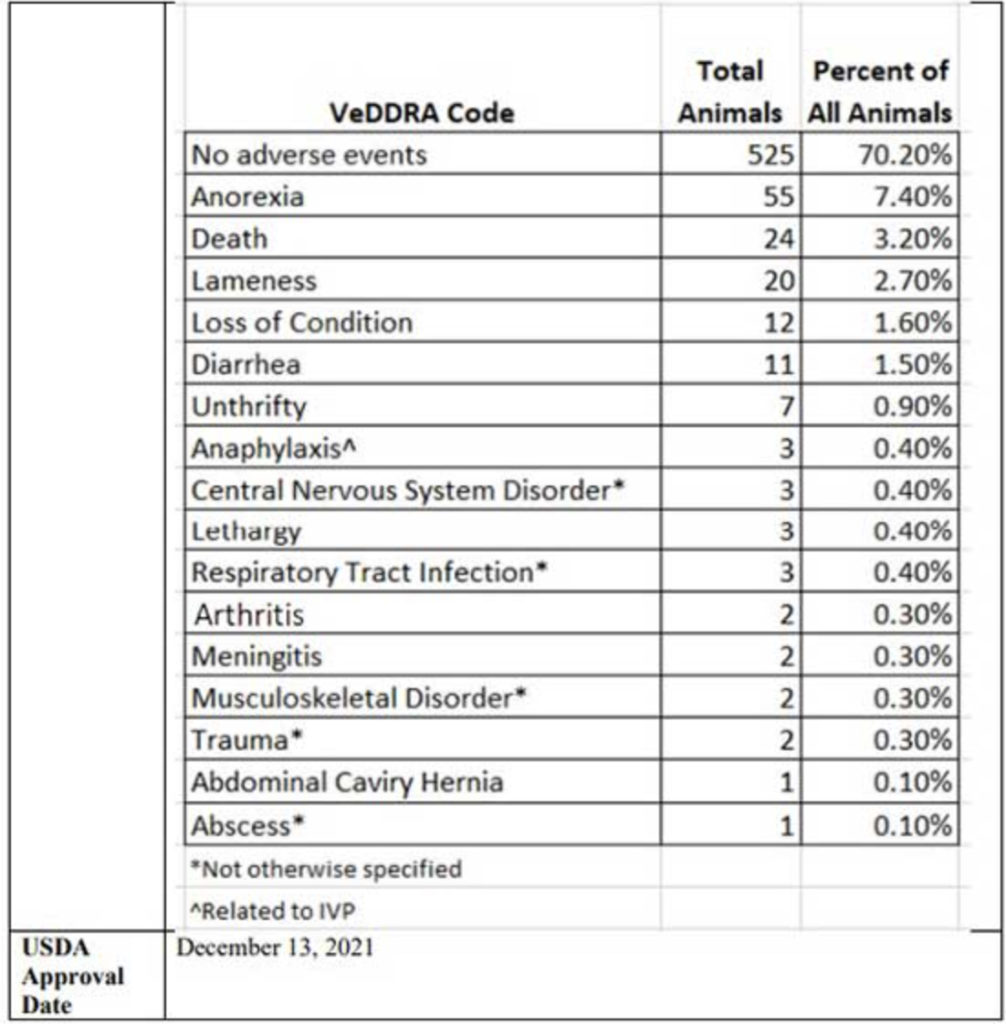
- 525 piglets — 70.2% — experienced no adverse events. The remaining 29.8% suffered a serious adverse event, including 24 deaths. When adding together death, anorexia (wasting) and unthrifty (failure to thrive), 11.5% of the animals were lost to vaccine injury
- The USDA license approval for Sequivity is very weak, and only includes data for influenza, even though Merck is advertising vaccines for four other pathogens under the Sequivity line. Reading through Canada’s Environmental assessment also makes it clear that safety claims are theoretical only
As previously reported, unbeknownst to the public, pork producers in the U.S.1 and Canada2 have been using customizable mRNA-based “vaccines” on their herds since 2018. As it turns out, the mRNA platform, Sequivity,3,4 is the only part of this gene-based “vaccine” technology that has been approved.
All customized mRNA injections created using this platform are untested, and the initial safety testing — upon which the assumed safety of all customized jabs are based — was grossly inadequate. As noted by Zoetis, the largest producer of veterinary drugs and vaccines:5
“Sequivity has safety and efficacy studies based on the platform with a historical initial isolate, not likely the isolate that customers would be requesting in their product.”
We don’t even know what that initial isolate was. The Sequivity platform allows customized “vaccines” to be created as follows, in as little as eight weeks:6
- Pathogen is collected and sent to a diagnostic lab.
- The gene of interest is sequenced and sent electronically to Sequivity analysts.
- A synthetic version of the gene of interest is synthesized and inserted into the RNA production platform.
- The RNA particles released from incubated production cells are harvested and formulated into a customized “vaccine.”
USDA Lists Only One Safety Study for Sequivity
So, just what kind of safety testing has been done on these mRNA swine jabs? As it turns out, not much, and even saying that would be an exaggeration. Looking at the U.S. Department of Agriculture’s (USDA) summary of studies supporting the product licensure of Sequivity,7 we find only ONE safety study listed.
There are two efficacy studies pertaining to H1N1 swine influenza, two efficacy studies pertaining to H1N2 swine influenza, one efficacy study pertaining to H3N2 swine influenza, and one safety study pertaining to all vaccines under “typical use conditions.”
These six studies were all performed between June 2020 and December 2021. A search of the USDA’s Licensed Veterinary Biological Products catalog renders just this one summary of studies to support Sequivity’s licensure.8 This implies the platform was approved by the USDA based on these data alone.
While that’s concerning enough, it becomes even more questionable when you consider the side effects summary. The study involved a total of 748 pigs at three different testing sites, which were given two doses of an unspecified mRNA Sequivity injection three weeks apart. The piglets were monitored for side effects for 21 days after each injection, or until the side effect was resolved.
According to the USDA summary, 525 piglets — 70.2% — experienced no adverse events. However, among the remaining 29.8%, 7.4% became anorexic, 3.2% died, 2.7% became lame, 0.4% suffered anaphylaxis, 0.4% had some sort of central nervous system disorder, 0.3% developed meningitis and another 0.3% some form of musculoskeletal disorder. For the complete list, see the graph below, taken from page 18 of the USDA’s Summary of Studies.9
In all, 223 out of 748 piglets — nearly 30% — had some form of adverse event, including 24 deaths. How on earth is that even remotely considered safe? If 3 in 10 people suffered a serious side effect from a particular drug, and over 3% dropped dead immediately and 11.5% in the long run, would you consider it safe? Would you risk taking it? I sure wouldn’t.
Also consider this. Anorexia is basically considered a wasting disease. “Unthrifty” is similar in that it’s the failure to grow or develop normally due to disease. Either way, whether the piglet is wasting or failing to grow, it’s a loss for the farmer. Now, when we add together death, anorexia and unthrifty, you end up with 86 animals — 11.5%.
Losing 11.5% of your herd to vaccine death, wasting and failure to thrive really doesn’t make sense. That’s a loss of more than 1 out of 10 animals, which hardly speaks to either safety or effectiveness. Moreover, there are no safety studies at all related to human consumption of Sequivity-treated animals.
No Large-Scale Safety Studies Were Ever Intended
Interestingly, large-scale safety studies don’t appear to ever have been part of the plan. As noted in the Canadian government’s environmental assessment of Merck’s RNA particle prescription products for swine influenza (now known as Sequivity), posted in July 2018:10
“The prescription products will not undergo large scale field safety testing … Merck Animal Health’s commercial SIV vaccine based on the RNA replicon particle platform was field safety tested in approximately 900 commercial pigs at three geographically separate locations in the USA …
Much of what is known about Merck Animal Health’s RNA replicon particle platform has been extrapolated from studies performed to characterize the company’s commercial vaccine: Swine Influenza Vaccine, RNA (USDA Product Code 19A5.D0, CCVB File 880VV/S3.0/H16).”
USDA Product Code 19A5.D0 refers to the very first RNA-based “vaccine” for swine, developed by Harrisvaccines and licensed in 2012.11,12,13 Harrisvaccines was acquired by Merck Animal Health in 2015 and 19A5.D0 became theirs.14,15
The Canadian environmental assessment also notes that the mRNA is encased in a lipid envelope originating from the VERO African green monkey kidney cell line — a continuous cell line shown to have tumorigenic potential16 (i.e., may encourage cancer growth). It also reviews how the virus is genetically modified before the RNA transcripts are purified. So, as with the COVID jab, the mRNA is not identical to what’s found in the natural pathogen. It’s modified.